NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Benznidazole is an orally available, broad spectrum antimicrobial agent used in the treatment of Chagas disease (American trypanosomiasis). Benznidazole is a nitroimidazole similar to metronidazole and is associated with serum enzyme elevations during therapy in up to 10% of patients but has not linked to cases of clinically apparent acute liver injury.
Background
Benznidazole (benz nid' a zole) is an oral, broad spectrum nitroimidazole antimicrobial that has activity against bacteria as well as several parasites. Like other nitroimidazoles such as metronidazole, benznidazole is activated intracellularly by bacterial or parasitic enzymes to a radical anion, which damages large protein molecules and DNA. Benznidazole has been found to be particularly effective against Trypanosoma cruzi, the cause of Chagas disease (American trypanosomiasis). In multiple clinical studies in both children and adults, with congenital, acute and chronic Chagas disease, a course of benznidazole has resulted in a high rate of cure. Chronic Chagas disease can lead to morbidity and mortality from digestive and cardiac disease and is endemic in much of Latin America. With increasing rates of emigration, Chagas disease has become a global problem and there are an estimated 300,000 persons with Chagas disease living in the United States. In 2017, benznidazole became the first agent to receive FDA approval as therapy for Chagas disease while nifurtimox became in second in 2020. Current indications for benznidazole are limited to children with Chagas disease ages 2 to 12 years. Benznidazole is available generically in tablets of 12.5 and 100 mg, and the recommended dose is 5 to 8 mg/kg orally in two divided doses daily for 60 days. Common side effects include abdominal pain, anorexia, weight loss, nausea, vomiting, headache, rash, urticaria and pruritus. Less common but potentially severe adverse reactions include hypersensitivity skin reactions, DRESS syndrome, peripheral neuropathy, bone marrow suppression, neutropenia, thrombocytopenia, anemia, carcinogenicity, genotoxicity and embryo-fetal toxicity.
Hepatotoxicity
Benznidazole therapy is associated with an appreciable rate of serum enzyme elevations, found in at least 10% of patients. The abnormalities, however, are generally mild, transient and without accompanying symptoms or jaundice. In clinical trials there were no reported instances of clinically apparent liver injury with jaundice attributed to benznidazole. However, since its approval and more widescale use, there have been several case reports of drug rash with eosinophilia and systemic symptoms (DRESS syndrome) accompanied by serum enzyme elevations associated with benznidazole therapy, one of which was accompanied by jaundice. Furthermore, cases of immunoallergic hepatitis have been reported with other more commonly used nitroimidazoles such as metronidazole and ornidazole, some of which have been severe. Thus, benznidazole therapy has had limited clinical use, but it appears to have the potential to cause symptomatic, immunoallergic hepatitis with jaundice.
Likelihood score: D (possible rare cause of clinically apparent liver injury, usually as a component of DRESS syndrome).
Mechanism of Injury
The cause of the serum enzyme elevations during benznidazole therapy is unknown. Benznidazole is metabolized in the liver where it undergoes glucuronidation but has not been found to lead to significant drug-drug interactions.
Outcome and Management
The severity of the liver injury linked to benznidazole therapy has been mild and self-limited, usually with transient, asymptomatic serum enzyme elevations, occasionally accompanied by hypersensitivity reactions or jaundice. However, the use of benznidazole has been limited. In instances of allergic reactions or anaphylaxis, benznidazole should be discontinued and rechallenge should be avoided. There is no information on whether there is cross sensitivity to hypersensitivity reactions or hepatic injury between benznidazole and other nitroimidazoles.
Drug Class: Antiinfective Agents, Trypanosomiasis Agents
Other Drugs in the Subclass, Nitroimidazoles: Fexinidazole, Metronidazole, Secnidazole, Tinidazole
Other Trypanosomiasis Agents: Fexinidazole, Nifurtimox
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Benznidazole – Generic
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Benznidazole | 22994-85-0 | C12-H12-N4-O3 |
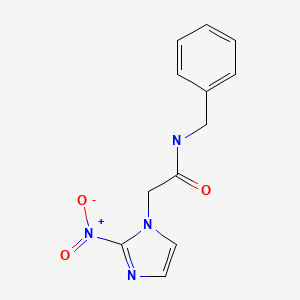
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 July 2023
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-637.(Expert review of hepatotoxicity of antibiotics published in 1999; does not mention benznidazole).
- Moseley RH. Antibacterial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013, p. 463-81.(Review of hepatotoxicity of antibacterial medications; does not discuss benznidazole).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections. In, Brunton LL, Halil-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 987-1000.(Textbook of pharmacology and therapeutics).
- Rosenthal PJ. Antiprotozoal drugs. In, Katzung BG, Masters SB, Trevor AJ. Basic and clinical pharmacology. 12th ed. New York: McGraw Hill, 2012, pp. 934.(Textbook of pharmacology and therapeutics mentions that benznidazole has activity similar to nifurtimox, the most frequently used drug for Chagas disease).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2017/209570Orig1s000SumR.pdf (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that ALT or AST elevations “were noted in all studies submitted for review” but were all transient and mostly mild and none were accompanied by jaundice). - CDC. Parasites - American trypanosomiasis (also known as Chagas disease). Available at: https://www
.cdc.gov/parasites/chagas/ (CDC website on American trypanosomiasis provides up-to-date information on epidemiology, diagnosis, clinical features, complications and therapy of Chagas disease and resources for obtaining nifurtimox and benznidazole). - Roe FJ. Safety of nitroimidazoles. Scand J Infect Dis Suppl 1985; 46: 72-81. [PubMed: 3865353](Review of the safety of the nitroimidazoles mentions that common side effects are nausea, headaches, metallic taste, furred tongue, itching and skin rash and occasional transient elevations in serum aminotransferase levels).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to a nitroimidazole antibiotic).
- Hasslocher-Moreno AM, do Brasil PE, de Sousa AS, Xavier SS, Chambela MC, Sperandio da Silva GM. Safety of benznidazole use in the treatment of chronic Chagas' disease. J Antimicrob Chemother 2012; 67: 1261-6. [PubMed: 22331592](Among 190 patients with chronic Chagas disease, ages 13 to 65 years, treated with benznidazole for 4-180 days, adverse events occurred in 49%, were severe in 0.5% and resulted in drug discontinuation in 15% of subjects, the major cause being skin reactions; no mention of ALT elevations or hepatotoxicity).
- Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 2014; 370: 1899-908. [PubMed: 24827034](Among 78 adults with chronic Chagas disease treated with benznidazole or posaconazole for 60 days, long term eradication of detectable parasites occurred in greater proportion of benznidazole recipients [62% vs 8-19%], while adverse events were less, including ALT elevations [20% vs 47%], ALT values above 5 times ULN occurring only in subjects on posaconazole).
- Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, et al.; BENEFIT Investigators. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 2015; 373: 1295-306. [PubMed: 26323937](Among 2854 South American patients with Chagas cardiomyopathy treated with benznidazole or placebo for up to 80 days, rates of parasite eradication were higher with treatment [66% vs 39.5%], but long term cardiac morbidity and mortality were not different; adverse events during treatment included skin reactions [9.6% vs 1.3%], gastrointestinal reactions [7.8% vs 2.9%] and peripheral neuropathy [3.6% vs 1.3%]; no mention of ALT elevations or hepatotoxicity).
- Bern C. Chagas' disease. N Engl J Med 2015; 373 (5): 456-66. [PubMed: 26222561](Review of the parasite life cycle, modes of transmission, epidemiology, clinical features, complications, diagnosis and treatment of Chagas disease; mentions that the most frequent side effect of benznidazole therapy is skin rash which, if accompanied by fever or lymphadenopathy, should lead to immediate discontinuation).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to metronidazole or other nitroimidazoles).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol 2015; 136: 1288-94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients receiving outpatient parenteral antibiotic therapy who had concurrent white blood cell and differential counts, eosinophilia [>500 cells/uL] was found in 25%, being most common with vancomycin [30%], the penicillins [28%], carbapenems [28%], cephalosporins [22%], fluoroquinolones [21%], and less common with metronidazole [15%], and there was no association of eosinophilia with liver test abnormalities).
- Manne-Goehler J, Umeh CA, Montgomery SP, Wirtz VJ. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis 2016; 10: e0005033. [PMC free article: PMC5098725] [PubMed: 27820837](Based upon rates of foreign born Hispanics in the population, there are 238,091 cases of Chagas disease in the US [largely unrecognized] and possibly another 109,000 among undocumented immigrants).
- Sperandio da Silva GM, Mediano MFF, Hasslocher-Moreno AM, Holanda MT, Silvestre de Sousa A, Sangenis LHC, Brasil PEAAD, et al. Benznidazole treatment safety: the Médecins Sans Frontières experience in a large cohort of Bolivian patients with Chagas' disease. J Antimicrob Chemother 2017; 72: 2596-601. [PubMed: 28645201](Among 2075 patients with Chagas disease treated with benznidazole in programs run by Doctors without Borders in Brazil, 56% had adverse events which were severe in 2 [0.1%] and resulted in discontinuation in 344 [16.5%], most of which were dermatologic reactions and 45% of whom were able to restart and finish therapy; no mention of ALT elevations or hepatotoxicity).
- Olivera MJ, Cucunubá ZM, Valencia-Hernández CA, Herazo R, Agreda-Rudenko D, Flórez C, Duque S, et al. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS One 2017; 12: e0185033. [PMC free article: PMC5614433] [PubMed: 28949997](Among 224 adults with Chagas disease treated with a 60 day course of benznidazole, side effects were common [91%] including rash [38%], itching [34%], abdominal pain [26%], bloating [24%] and nausea [27%], leading to early discontinuation in 23% of patients; eosinophilia arose in 9%, and DRESS in 21%, while ALT or AST were elevated in 2% of patients, but all adverse events resolved upon stopping).
- Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, Silva JLP, et al. Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS Negl Trop Dis 2018; 12: e0006814. [PMC free article: PMC6211620] [PubMed: 30383777](Among 1813 Brazilian patients with Chagas disease and abnormal electrocardiograms who were followed for at least 2 years, the mortality rate was lower in the 493 who had received at least one course of benznidazole than in those who did not [2.8% vs 7.8%] as were rates of parasitemia [17% vs 36%] and EKG abnormalities [48% vs 60%]; no mention of ALT elevations or hepatotoxicity).
- Pavan TBS, Silva JWD, Martins LC, Costa SCB, Almeida EA. Hepatic changes by benznidazole in a specific treatment for Chagas disease. PLoS One 2018; 13: e0200707. [PMC free article: PMC6054377] [PubMed: 30028842](Among 204 patients with Chagas disease [7% acute, 93% chronic] treated with benznidazole, 48 [24%] had skin rash and 35 [17%] had serum enzyme elevations [peak ALT 310 U/L, AST 220 U/L, Alk P 302 U/L and GGT 258 U/L]; no mention of jaundice or outcome of the abnormalities).
- Moreno-Escobosa C, Cruz-Granados S. Drug reaction with eosinophilia and systemic symptoms syndrome induced by benznidazole. Contact Dermatitis 2018; 79: 105-6. [PubMed: 29635801](30 year old Bolivian woman with chronic Chagas disease developed fever, rash, eosinophilia and serum ALT elevations [103 U/L] 50 days after starting benznidazole and while also receiving cloxacillin, naproxen and dipyrone).
- Meymandi S, Hernandez S, Park S, Sanchez DR, Forsyth C. Treatment of Chagas disease in the United States. Curr Treat Options Infect Dis 2018; 10: 373-88. [PMC free article: PMC6132494] [PubMed: 30220883](Review of the epidemiology, clinical features, diagnosis, complications and therapy of Chagas disease as seen in the US; mentions that reported response rates to benznidazole are 96% for congenital, 76% for acute, 62% of chronic infection in children and 37% for chronic infection in adults; recommends routine liver tests before starting therapy and regular monitoring during treatment).
- Crespillo-Andújar C, Venanzi-Rullo E, López-Vélez R, Monge-Maillo B, Norman F, López-Polín A, Pérez-Molina JA. Safety profile of benznidazole in the treatment of chronic Chagas disease: experience of a referral centre and systematic literature review with meta-analysis. Drug Saf 2018; 41: 1035-48. [PubMed: 30006773](Systematic review of 42 studies [7822 patients] of safety of benznidazole in Chagas disease found overall rate of adverse events of 44% but range of 0-98% and while there was no mention of specific symptoms or ALT elevations, serious adverse events mentioned included “toxic hepatitis” without further details).
- Ataee P, Karimi A, Eftekhari K. Hepatic failure following metronidazole in children with Cockayne syndrome. Case Rep Pediatr 2020; 2020: 9634196. [PMC free article: PMC7008298] [PubMed: 32082677](Two cases: 2 and 5 years old, both boys with type B [infant onset] Cockayne syndrome developed jaundice, vomiting and agitation 2 and 3 days after starting oral metronidazole for minor infection [bilirubin 13.7 and 16.0 mg/dL, ALT 2815 and 2248 U/L, Alk P 1265 and 1654 U/L, INR 5.1 and 4.6], one dying within 2 days of admission and the second recovering but with persistent jaundice and serum enzyme elevations one month later; whether benznidazole might cause similar injury is not known).
- Giancola ML, Corpolongo A, Comandini UV, Del Nonno F, Montalbano M, Petrone A, Carrara S, et al. Severe drug-induced liver injury (DILI) associated with benznidazole therapy for Chagas' disease. J Antimicrob Chemother. 2022;77:3515-3517. [PubMed: 36173378](68 year old woman with chronic indeterminate Chagas disease developed rash 3 weeks after starting benznidazole with elevations in ALT [178 rising to 594 U/L], but normal bilirubin and alkaline phosphatase levels at 5 weeks when benznidazole was stopped and she was treated with high dose methylprednisolone and 2-3 weeks later had further rise in liver test abnormalities [ALT 3170 U/L, bilirubin 4.1 mg/dL], which resolved spontaneously over the next month).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Secnidazole.[LiverTox: Clinical and Researc...]Review Secnidazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Tinidazole.[LiverTox: Clinical and Researc...]Review Tinidazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- An evaluation of benznidazole as a Chagas disease therapeutic.[Expert Opin Pharmacother. 2019]An evaluation of benznidazole as a Chagas disease therapeutic.Caldas IS, Santos EG, Novaes RD. Expert Opin Pharmacother. 2019 Oct; 20(15):1797-1807. Epub 2019 Aug 28.
- Characteristics of Patients for Whom Benznidazole Was Released Through the CDC-Sponsored Investigational New Drug Program for Treatment of Chagas Disease - United States, 2011-2018.[MMWR Morb Mortal Wkly Rep. 2018]Characteristics of Patients for Whom Benznidazole Was Released Through the CDC-Sponsored Investigational New Drug Program for Treatment of Chagas Disease - United States, 2011-2018.Herwaldt BL, Dougherty CP, Allen CK, Jolly JP, Brown MN, Yu P, Yu Y. MMWR Morb Mortal Wkly Rep. 2018 Jul 27; 67(29):803-805. Epub 2018 Jul 27.
- Review Nifurtimox.[LiverTox: Clinical and Researc...]Review Nifurtimox.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Benznidazole - LiverToxBenznidazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...