NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The second generation sulfonylureas include glyburide (also known as glibenclamide), gliclazide, glipizide, and glimepiride, which are oral hypoglycemic agents that are widely used in therapy of type 2 diabetes. These agents are known, but infrequent causes of clinically apparent liver injury.
Background
The sulfonylureas are substituted arylsulfonylureas, their differences being in the types of substitutions at the two ends of the molecule. The sulfonylureas lower blood glucose through an increase in secretion of insulin from pancreatic beta cells. They may also have other extra-pancreatic hypoglycemic actions that are important during prolonged therapy. The second generation sulfonylureas have largely replaced the first generation agents in routine use, because they are more potent, can be administered in lower doses, and can be given on a once daily basis. These agents are recommended as a first line of therapy for type 2 diabetes, but should be administered in conjunction with advice on diet and exercise. The sulfonylureas can also be used in combination with other hypoglycemic agents such as metformin, pioglitazone, alpha glucosidase inhibitors, incretin based drugs or insulin. Because of the similarity of mechanism of action, the sulfonylureas are not recommended to be used in combination with the metiglinides such as nateglinide and repaglinide. Common side effects of the second generation sulfonylureas include headache, dizziness, paresthesias, abdominal discomfort, nausea and weight gain. These agents are also associated with an “antabuse” like response to alcohol (although less likely that with first generation sulfonylureas) and patients should be advised not to drink alcoholic beverages. All sulfonylureas can cause hypoglycemia. The second generation sulfonylureas are all labelled with a special warning about increased risk for cardiovascular mortality.
Glyburide (glye' bure ide), which is known in other countries as glibenclamide, is available in generic forms and under the brand name of DiaBeta and Micronase in tablets of 1.25, 2.5 and 5 mg, the recommended dose initially being 1.25 to 5 mg daily, with increases based upon blood glucose and tolerance to a maximum of 20 mg daily, either in a single or divided dose. Micronized formulations are also available in tablets of 1.5, 3 and 6 mg, that may provide more sustained release. Fixed combinations of glyburide with metformin are also available in many generic forms and under the commercial name Glucovance.
Gliclazide is available in generic forms and under the brand name of Diamicron and Dianorm in many countries, but not in the United States. The recommended dose initially is 40 to 80 mg twice daily, with increases weekly to achieve adequate glycemic control. The maximum dose is 320 mg daily.
Glimepiride (glye mep' ir ide) is available under in several generic forms and the brand name of Amaryl in tablets of 1, 2 and 4 mg, the recommended dose initially being 1 to 2 mg once daily, with increases based upon blood glucose and tolerance to a maximum of 8 mg once daily. Fixed dose combinations of glimepiride with thiazolidinediones have also been marketed (with rosiglitazone as Avandaryl, and with pioglitazone as Duetact).
Glipizide (glip' i zide) is available in multiple generic forms and under the brand name of Glucotrol in tablets of 5 and 10 mg and as an extended release form (Glucotrol XL) in 2.5, 5 and 10 mg, the recommended dose initially being 5 to 10 mg daily, with increases based upon blood glucose and tolerance to a maximum of 15 mg (standard formulation) or 20 mg (extended release formulation) once daily. Higher doses should be given in divided doses daily to a daily maximum of 40 mg. Fixed combinations of glipizide with metformin are also available in both generic and brand name forms (Metaglip).
Hepatotoxicity
Minor enzyme elevations have been reported to occur during sulfonylurea therapy in less than 1% of patients, rates that are similar to what occurs with placebo therapy. Clinically apparent liver injury from the sulfonylureas is rare, but has been reported for virtually all of the currently available forms and appears to be a class effect. The liver injury appears within 3 to 12 weeks of starting the medication, usually with symptoms of fatigue, nausea and abdominal discomfort, followed by dark urine and jaundice. Rare instances of hepatic injury arising after many months or years of therapy have been reported, particularly soon after an increase in dosage. Various combinations of hepatocellular and cholestatic injury have been described with sulfonylurea induced liver injury, and many cases are reported to be mixed. Allergic manifestations can occur, but are not typical and autoantibody formation is rare. Because sulfonylureas are usually given in combination with other hypoglycemic agents, many of which also cause liver injury, it can be difficult to establish whether the injury is due to the sulfonylurea or another agent. The timing of injury in relationship to starting the medication is perhaps most characteristic feature. Recovery is usually rapid once the medication is stopped. While all of the second generation sulfonylureas have been linked to cases of clinically apparent liver injury, the most cases have been associated with glyburide (referred to as glibenclamide outside of the United States), perhaps because it has been the most widely used.
Glyburide likelihood score: B (likely rare cause of clinically apparent liver injury).
Gliclazide likelihood score: D (possible cause of clinically apparent liver injury).
Glimepiride likelihood score: C (probable cause of clinically apparent liver injury).
Glipizide likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of liver injury due to sulfonylureas is unknown, but suspected to be due to hypersensitivity. Cross reactivity to reactions to sulfonamides can occur, but not invariably and the overall pattern of injury and outcome of sulfonylurea associated hepatic injury does not resemble the acute, immunoallergic pattern that is common with the sulfonamides. Nevertheless, the sulfonylureas should be used with caution in patients with sulfonamide hypersensitivity or a history of sulfonamide-hepatotoxicity. In either case, rechallenge usually leads to recurrence and should be avoided.
Outcome and Management
The liver injury from the sulfonylureas usually resolves rapidly once the agent is stopped, but chronic injury has been described in rare cases. Despite the usual cholestatic form of injury, cases of progressive hepatic failure and death have been described, particularly in the elderly or patients with multiple other medical problems and when the medication is not promptly withdrawn. Sulfonylureas are rarely listed in causes of acute liver failure due to medications. In cases in which multiple hypoglycemic agents were being used when the liver injury appears, rechallenge with the agent least likely associated with the injury is appropriate.
References to the second generation sulfonylureas and examples of hepatotoxicity are given in the Overview section on the Sulfonylureas (updated March 2018).
Drug Class: Antidiabetic Agents
Other Drugs in the Subclass, Sulfonylureas: First Generation Sulfonylureas
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gliclazide – Diamicron®
Glimepiride – Amaryl®
Glipizide – Glucotrol®
Glyburide – Diabeta®, Micronase®
DRUG CLASS
Antidiabetic Agents
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Gliclazide | 21187-98-4 | C15-H21-N3-O3-S |
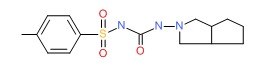 |
| Glimepiride | 93479-97-1 | C24-H34-N4-O5-S |
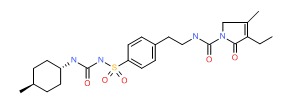 |
| Glipizide | 29094-61-9 | C21-H27-N5-O4-S |
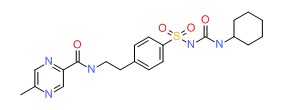 |
| Glyburide | 10238-21-8 | C23-H28-Cl-N3-O5-S |
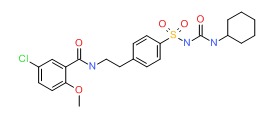 |
- PubChem SubstanceRelated PubChem Substances
- Pharmacokinetics of gliquidone, glibenclamide, gliclazide and glipizide in middle-aged and aged subjects.[Res Commun Mol Pathol Pharmaco...]Pharmacokinetics of gliquidone, glibenclamide, gliclazide and glipizide in middle-aged and aged subjects.Courtois P, Sener A, Herbaut C, Turc A, Malaisse WJ. Res Commun Mol Pathol Pharmacol. 1999 Feb; 103(2):211-22.
- Review The place of gliclazide MR in the evolving type 2 diabetes landscape: A comparison with other sulfonylureas and newer oral antihyperglycemic agents.[Diabetes Res Clin Pract. 2018]Review The place of gliclazide MR in the evolving type 2 diabetes landscape: A comparison with other sulfonylureas and newer oral antihyperglycemic agents.Colagiuri S, Matthews D, Leiter LA, Chan SP, Sesti G, Marre M. Diabetes Res Clin Pract. 2018 Sep; 143:1-14. Epub 2018 May 24.
- Review Sulfonylureas.[LiverTox: Clinical and Researc...]Review Sulfonylureas.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- The risk of overall mortality in patients with Type 2 diabetes receiving different combinations of sulfonylureas and metformin: a retrospective analysis.[Diabet Med. 2012]The risk of overall mortality in patients with Type 2 diabetes receiving different combinations of sulfonylureas and metformin: a retrospective analysis.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Nutter B, Jain A, Atreja A, Zimmerman RS. Diabet Med. 2012 Aug; 29(8):1029-35.
- Pharmacologic Differences of Sulfonylureas and the Risk of Adverse Cardiovascular and Hypoglycemic Events.[Diabetes Care. 2017]Pharmacologic Differences of Sulfonylureas and the Risk of Adverse Cardiovascular and Hypoglycemic Events.Douros A, Yin H, Yu OHY, Filion KB, Azoulay L, Suissa S. Diabetes Care. 2017 Nov; 40(11):1506-1513. Epub 2017 Sep 1.
- Sulfonylureas, Second Generation - LiverToxSulfonylureas, Second Generation - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...