NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sulindac is a commonly used nonsteroidal antiinflammatory drug (NSAID) that is available by prescription only and used predominantly to treat chronic arthritis. Sulindac is a rare, but well established cause of idiosyncratic, clinically apparent drug induced liver disease.
Background
Sulindac (sul' in dak) is a member of the indene acetic acid class of NSAIDs and is chemically related to indomethacin. Like other NSAIDs, sulindac acts through inhibition of tissue cyclooxygenases (Cox-1 and Cox-2) which leads to a decrease in synthesis of proinflammatory prostaglandins, potent mediators of pain and inflammation. Sulindac has analgesic as well as antipyretic and antiinflammatory activities. Sulindac was approved for use in chronic arthritis in the United States in 1978 and its indications have been expanded since. Current indications include acute and chronic use for osteoarthritis, rheumatoid arthritis, anklyosing spondylitis, acute gouty arthritis and for acute bursitis. When given chronically, sulindac has also been shown to decrease adenoma formation in persons with familial adenomatous polyposis. Generic formulations are available (150 and 200 mg) and specific commercial names include Clinoril (100, 150, 200 mg). The recommended dose in adults is 150 to 200 mg twice daily. As with other NSAIDs, sulindac is generally well tolerated, but side effects can include headache, dizziness, somnolence, gastrointestinal upset, nausea, abdominal discomfort, diarrhea, peripheral edema and hypersensitivity reactions. Rare but serious adverse events from NSAIDs include gastrointestinal ulceration and bleeding, increased risk for cardiovascular disease, renal dysfunction and hypersensitivity reactions including anaphylaxis, exfoliative dermatitis and Stevens Johnson syndrome.
Hepatotoxicity
Chronic therapy with sulindac is associated with a low rate of serum aminotransferase elevations, which are rarely severe and usually self-limited. Clinically apparent acute liver injury from sulindac is well known, but rare (~5 cases in 100,000 prescriptions and ~0.1% of users). Sulindac hepatotoxicity typically presents with fever, rash, nausea and vomiting and abdominal pain arising within a few days or weeks of starting the medication and followed shortly thereafter by jaundice. Occasionally, the onset may be delayed, particularly if therapy is intermittent. The clinical pattern suggests an allergic hepatitis and is somewhat similar to the hepatotoxicity of the sulfonamides. The pattern of serum enzyme elevations is usually hepatocellular or mixed at the onset, but may then become cholestatic. However, recovery is usually rapid once sulindac is stopped. Histology is consistent with an allergic hepatitis with spotty necrosis and marked inflammatory cell infiltration with prominence of eosinophils. In many instances, the features of hypersensitivity (such as facial swelling, desquamating rash, pharyngitis, stomatitis, lymphadenopathy, and hypotension) overshadow the liver injury and are more commonly the cause of death. Sulindac can also cause acute liver injury with a more delayed latency with few or no features of hypersensitivity. These cases are usually cholestatic and can be prolonged and lead to vanishing bile duct syndrome.
Likelihood score: A (well established, although rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of sulindac hepatotoxicity appears to be hypersensitivity, since features of immunoallergic hepatitis are common, occurring in at least half of patients. Cases not accompanied by hallmarks of hypersensitivity tend to appear after longer periods of exposure and suggest a possible metabolic, rather than immunologic idiosyncrasy.
Outcome and Management
The minor aminotransferase elevations that occur in a small proportion of patients during chronic therapy with sulindac are usually subclinical and not progressive. The clinically apparent liver injury from sulindac ranges in severity from mild, self-limited immunoallergic hepatitis, to a prolonged and severe cholestasis to acute liver failure and death. Prognosis may relate, at least in part, to whether sulindac is discontinued promptly and to whether other organs are involved in a hypersensitivity reaction. If hypersensitivity features are prominent or liver injury is severe, corticosteroid therapy may be appropriate, although such intervention has not been proven to be beneficial. Complete recovery with normalization of laboratory abnormalities usually occurs within 1 to 2 months, but can take up to half a year after stopping sulindac. In cases of cholestatic liver injury arising later during sulindac therapy, recovery is often more delayed and instances of vanishing bile duct syndrome have been reported. After immunoallergic liver injury, reexposure to sulindac usually results in rapid recurrence of liver injury and allergic features which can be severe. Thus, rechallenge with sulindac should be avoided. There is rarely cross reactivity in liver injury between sulindac and NSAIDs of other classes, but there may be cross reactivity to allergic reactions to indomethacin and the sulfonamides.
Drug Class: Nonsteroidal Antiinflammatory Drugs
CASE REPORT
Case 1. Severe immunoallergic hepatitis attributed to sulindac.(1)
A 51 year old woman with a chronic myeloproliferative disorder developed nausea, fatigue and fever within 24 hours of starting sulindac for back pain. She had no history of liver disease, had no risk factors for viral hepatitis, did not drink alcohol, and was taking no other medications. Physical examination revealed mild jaundice and fever; there was no mention of rash. Initial laboratory tests showed bilirubin of 3.7 mg/dL and 10-fold elevations of ALT with minimal increases in alkaline phosphatase (Table). Tests for hepatitis B and autoantibodies were negative. Ultrasound showed hepatosplenomegaly without biliary tract abnormalities. She recovered rapidly and was well during follow up until she inadvertently restarted sulindac and redeveloped symptoms of fatigue, fever and jaundice within 24 hours. On readmission, she was markedly jaundiced and serum ALT levels were 100-fold elevated. A liver biopsy taken 2 to 3 weeks later showed evidence of hepatocellular necrosis, cholestasis and drug induced liver disease. She recovered rapidly and laboratory tests were near normal within 8 weeks.
Key Points
| Medication: | Sulindac (150 mg orally taken twice daily for one day) |
|---|---|
| Pattern: | Hepatocellular (R=9 initially; >100 on rechallenge, later falling to cholestatic range, with R=2.6 and <1.0) |
| Severity: | 4+ (hospitalized, jaundiced, prolongation of prothombin time) |
| Latency: | 1 day to symptoms, 3 days to jaundice |
| Recovery: | 4 to 8 weeks |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 0 | Two doses of sulindac (200 mg) taken | ||||
| 1 day | 0 | 317 | 275 | 3.7 | |
| 4 days | 3 days | 307 | 687 | 2.7 | |
| 9 days | 8 days | 180 | 562 | 1.4 | |
| 19 days | 18 days | 23 | 14 | 1.3 | |
|
Time After
Restarting |
Time After
Restopping | 5 months later, two doses of sulindac taken | |||
| 5 days | 4 days | 4060 | |||
| 8 days | 7 days | 835 | 276 | 21.0 | Protime=18.3 sec |
| 11 days | 10 days | 395 | 18.0 | ||
| 16 days | 15 days | 71 | 407 | 11.0 | |
| 19 days | 18 days | 39 | 508 | 6.0 | |
| 4 weeks | 4 weeks | 30 | 499 | 3.0 | Liver biopsy |
| 10 weeks | 10 weeks | 29 | 290 | 1.2 | |
| Normal Values | <35 | <275 | <1.2 | ||
Comment
This patient developed an acute immunoallergic hepatitis that was attributed to sulindac, and the inadvertent rechallenge made the association definite. Upon initial presentation, the pattern of enzyme elevations was hepatocellular, but the pattern rapidly became cholestatic over the course of the illness and a liver biopsy was described as showing a cholestatic hepatitis. The patient reported allergy to house dust and pollen, but there was no history of previous drug-allergy. In patients with hypersensitivity reactions to sulindac, it is best to avoid other indene acetic acid NSAIDs such as indomethacin and possibly appropriate to avoid use of sulfonamides. The case history is a strong reminder that patients with drug induced liver disease should be told to discard any remaining pills of the implicated medication.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sulindac – Generic, Clinoril®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sulindac | 38194-50-2 | C20-H17-F-O3-S |
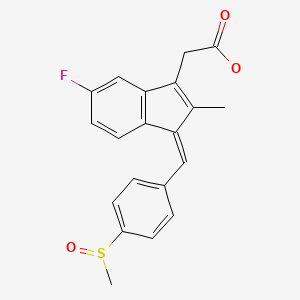
|
CITED REFERENCE
- 1.
- Daniele B, Pignata S, D’Agostino L, Vecchione R, Mazzacca G. Sulindac-induced severe hepatitis. Am J Gastroenterol. 1988;83:1429–31. [PubMed: 3195554]
ANNOTATED BIBLIOGRAPHY
References updated: 20 March 2020
Abbreviations: NSAIDs, nonsteroidal antiinflammatory drugs.
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-53.(Expert review of hepatotoxicity published in 1999; sulindac has been implicated in 25 published cases of liver injury with jaundice and 91 reported to the FDA, typically with cholestatic or mixed enzyme elevations and a 5% fatality rate, but usually due to generalized hypersensitivity reactions).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp. 370-402.(Expert review of hepatotoxicity of NSAIDs mentions that sulindac is one of the most commonly implicated NSAIDs in causing liver injury, typically within 1-8 weeks of starting the drug and with a cholestatic pattern of injury with features of hypersensitivity).
- Grossner T, Smyth EM, Fitzgerald GA. Pharmacotherapy of inflammation, fever, pain, and gout. In, Brunton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th ed. New York: McGraw-Hill, 2018. pp. 685-709.(Textbook of pharmacology and therapeutics).
- Anderson RJ. Severe reaction associated with sulindac administration. N Engl J Med. 1979;300:735–6. [PubMed: 763309](18 year old woman developed nausea, facial edema, rash and fever 7 days after starting sulindac [bilirubin 9.8 mg/dL, ALT and Alk P "off the scale"], with coagulopathy and thrombocytopenia, resolving on corticosteroid therapy and within 3 weeks of stopping sulindac).
- Wolfe PB. Sulindac and jaundice. Ann Intern Med. 1979;91:656. [PubMed: 484983](46 year old woman developed jaundice 2 weeks after restarting sulindac [bilirubin 10.2 mg/dL, AST 259 U/L, Alk P >350 U/L, GGTP 1897 U/L], resolving within 2 months of stopping).
- Smith FE, Lindberg PJ. Life-threatening hypersensitivity to sulindac. JAMA. 1980;244:269–70. [PubMed: 7382094](18 year old woman with rheumatoid arthritis developed high fever and sore throat 5 days after starting sulindac [bilirubin not given; AST 350 U/L, Alk P 227 U/L], resolving in 10 days; recurrence of fever and hypotension 4 hours after rechallenge with a single dose).
- Dhand AK, LaBrecque DR, Metzger J. Sulindac (Clinoril) hepatitis. Gastroenterology. 1981;80:585–6. [PubMed: 7450449](29 year old woman with systemic lupus erythematosus developed fever, rash, arthralgias and nausea 18 days after starting sulindac [bilirubin 2.2 mg/dL, ALT >350 U/L, Alk P 227 U/L], with rapid recovery on stopping; rapid recurrence of high fever, rash and abnormal laboratory tests on reexposure).
- Kaul A, Reddy JC, Fagman E, Smith GF. Hepatitis associated with use of sulindac in a child. J Pediatr. 1981;99:650–1. [PubMed: 7277113](12 year old girl developed fever, nausea, abdominal pain and jaundice 6 weeks after starting sulindac, with rapid recovery, but immediate and severe recurrence [bilirubin 6.5 mg/dL, ALT 67 U/L, Alk P 930 U/L, GGT 815 U/L] within 24 hours of single tablet rechallenge).
- McIndoe GA, Menzies KW, Reddy J. Sulindac (Clinoril) and cholestatic jaundice. N Z Med J. 1981;94:430–1. [PubMed: 6950284](54 year old woman developed jaundice 5 days after completion of a 7 day course of sulindac [bilirubin 5.0 mg/dL, ALT 79 U/L, Alk P 223 U/L]; biopsy showed intrahepatic cholestasis; rapid resolution).
- Giroux Y, Moreau M, Kass TG. Cholestatic jaundice caused by sulindac. Can J Surg. 1982;25:334–5. [PubMed: 7083082](66 year old woman developed jaundice 3 months after starting sulindac [bilirubin 6.9 rising to 20 mg/dL, ALT 328 U/L, Alk P 3 times ULN], resolving in 4 months and recurrence of jaundice within 3 days of rechallenge).
- Kammerer J, Sabardeil S, Rumeau JL, Salson A, El Hage A. Gastroenterol Clin Biol. 1982;6:712–3. [Hepatitis due to Sulindac; a case with review of 3 precedents] French. [PubMed: 7129022](79 year old woman developed fever, arthralgias, rash and jaundice 2 weeks after restarting sulindac which had been used intermittently for 6 months [bilirubin 10.4 mg/dL, ALT 650 U/L, Alk P 5 times ULN]; cholestatic hepatitis on biopsy and rapid resolution upon stopping).
- Park GD, Spector R, Headstream T, Goldberg M. Serious adverse reactions associated with sulindac. Arch Intern Med. 1982;142:1292–4. [PubMed: 6212034](Case series of 4 patients with severe hypersensitivity reactions arising within 5 to 30 days of starting sulindac with variety of organ involvement: skin and liver most common, but also lung, central nervous system, lymph nodes, and bone marrow).
- Whittaker SJ, Amar JN, Wanless IR, Heathcote J. Sulindac hepatotoxicity. Gut. 1982;23:875–7. [PMC free article: PMC1419834] [PubMed: 7117907](Two women, ages 70 and 71 years, developed jaundice 5 and 30 days after starting sulindac [bilirubin 3.2 and 12.5 mg/dL, AST 211 and 65 U/L, Alk P 325 and 278 U/L], both recovering rapidly upon stopping).
- Fagan EA, Walford N, Hodgson HJ. Sulindac hepatotoxicity. Gut. 1983;24:1199. [PMC free article: PMC1420262] [PubMed: 6642281](Letter in response to Whittaker [1982] describing a 45 year old woman who developed jaundice 3 months after starting sulindac [bilirubin 11.6 mg/dL, AST 246 U/L, Alk P 595 U/L], resolving within 12 weeks of stopping).
- Klein SM, Khan MA. Hepatitis, toxic epidermal necrolysis and pancreatitis in association with sulindac therapy. J Rheumatol. 1983;10:512–3. [PubMed: 6224935](52 year old woman developed fever, rash and fatigue 14 days after starting sulindac with oral ulcers and facial edema [Stevens-Johnson syndrome] [bilirubin 7.0 mg/dL, AST 590 U/L, Alk P not given], with multiorgan failure and death in 5 days. A second case of 57 year old woman who developed pancreatitis [normal liver tests] 3 months after starting sulindac, recurring rapidly on reexposure).
- Bunde B, Deckers Y, Dequeker J. Fentiazac in rheumatoid arthritis: comparison with sulindac and long-term tolerance. Curr Med Res Opin. 1983;8:310–4. [PubMed: 6340972](Long term therapy with fentiazac in 33 patients; 3 developed hepatotoxicity after 4 months with bilirubin normal to 7.5 mg/dL, ALT 86 to 1040 U/L, Alk P 152 to 290, with rapid resolution after stopping).
- Lewis JH. Hepatic toxicity of nonsteroidal anti-inflammatory drugs. Clin Pharm. 1984;3:128–38. [PubMed: 6373099](Review of hepatotoxicity of NSAIDs; sulindac reported to cause cholestatic or mixed pattern of injury with hypersensitivity features).
- Bodin F, Habibi B, Legendre C, Schaeffer-Plumet J, Riallin P, Darnis F. Gastroenterol Clin Biol. 1985;9:546–7. [Hepatotoxicity of sulindac. A new case] French. [PubMed: 4018492](79 year old woman developed nausea, jaundice and urticaria 4 days after starting sulindac [bilirubin 12 mg/dL, ALT 25 times ULN, Alk P 1.5 times ULN, anti-sulindac antibodies], with eosinophilia and thrombocytopenia, resolving rapidly).
- Gallanosa AG, Spyker DA. Sulindac hepatotoxicity: a case report and review. J Toxicol Clin Toxicol. 1985;23:205–38. Erratum in: J Toxicol Clin Toxicol 1985-86; 23: 617. [PubMed: 3903180](44 year old woman developed abdominal pain with mild ALT [293 U/L] and Alk P [136 U/L] elevations after 6 months of intermittent sulindac therapy, resolving with stopping, but while taking multiple other drugs).
- Wood LJ, Mundo F, Searle J, Powell LW. Sulindac hepatotoxicity: effects of acute and chronic exposure. Aust N Z J Med. 1985;15:397–401. [PubMed: 3866535](3 cases: 57 year old woman developed rash, sore throat, malaise and fever 6 days after starting sulindac [bilirubin 1.0 mg/dL, AST 97 U/L, Alk P 450 U/L], resolving within 7 weeks of stopping; 67 year old man developed rash and pruritus 4 weeks after starting sulindac [bilirubin 1.9 mg/dL, AST 41 U/L, Alk P 450 U/L], persisting while drug was continued and resolving within 1 month of stopping; 42 year old woman developed fatigue and rash 3 weeks after starting sulindac [bilirubin 1.0 mg/dL, AST 230 U/L, Alk P 208 U/L], resolving within 2 months of stopping).
- [Sulindac—a review of adverse effects. Liver reactions are the most characteristics] Lakartidningen 1987; 84: 142, 144. Swedish. [PubMed: 3821321](Among adverse events reports to a Swedish registry due to sulindac made between 1982-1986 were 44 cases of liver injury, presenting 2 to 12 weeks after starting sulindac and typically with a mixed pattern of serum enzyme elevations).
- Lerche A, Vyberg M, Kirkegaard E. Acute cholangitis and pancreatitis associated with sulindac (clinoril). Histopathology. 1987;11:647–53. [PubMed: 3623432](68 year old man with recurrent attacks of nausea, abdominal pain and fever who was taking sulindac intermittently; selective challenge let to rapid appearance of fever, pain, and nausea [amylase 1542 U/L, ALT 80 U/L, Alk P 400 U/L without jaundice], liver biopsy showing non-specific changes).
- Daniele B, Pignata S, D'Agostino L, Vecchione R, Mazzacca G. Sulindac-induced severe hepatitis. Am J Gastroenterol. 1988;83:1429–31. [PubMed: 3195554](51 year old woman developed nausea, malaise and fever after 2 doses of sulindac with subsequent jaundice [bilirubin 3.6 mg/dL, ALT 317 U/L, Alk P 275 U/L], rapid recovery; inadvertent readministration led to recurrence within 2 days [bilirubin rising to 21 mg/dL, ALT 4060 U/L and Alk P 276 U/L], resolving within 2 months: Case 1).
- Hannequin JR, Doffoel M, Schmutz G. Rev Rhum Mal Osteoartic. 1988;55:983–8. [Hepatitis secondary to current non-steroidal anti-inflammatory agents] French. [PubMed: 3070713](Review of the literature on hepatotoxicity of NSAIDs; 21 cases were attributed to sulindac, occurring at all ages, with latency to onset of 4 days to 2 months, commonly with jaundice and fever and often with rash, one fatality, several instances of recurrence with reexposure suggesting an immunoallergic basis).
- Kromann-Andersen H, Pedersen A. Reported adverse reactions to and consumption of nonsteroidal anti-inflammatory drugs in Denmark over a 17-year period. Dan Med Bull. 1988;35:187–92. [PubMed: 2966038](Summary of 2721 adverse event reports on NSAIDs from 1969-85; hepatic injury in 3% of reports [3 fatal]; rates of hepatic adverse drug reactions per million daily doses was highest for sulindac [1.2] compared to diclofenac [0.4], tolmetin [0.3] phenylbutazone [0.2], ibuprofen, naproxen, ketoprofen or indomethacin [0.1]).
- Tarazi EM, Harter JG, Zimmerman HJ, Ishak KG, Eaton RA. Sulindac-associated hepatic injury: analysis of 91 cases reported to the Food and Drug Administration. Gastroenterology. 1993;104:569–74. [PubMed: 8425699](Analysis of reports of sulindac hepatotoxicity submitted to FDA between 1978 and 1986: among 91 cases, 50% presented within 4 weeks, 65% were women, 67% had jaundice, 50% cholestatic enzymes, 55% fever, 48% rash, 40% pruritus, 35% eosinophilia, and 5% died, mostly from hypersensitivity reaction).
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. [PubMed: 8385741](Small trial showing decrease in adenoma formation in high risk subjects on sulindac therapy; no patient had serious side effects or liver enzyme elevations).
- García Rodríguez LA, Williams R, Derby LE, Dean AD, Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Intern Med. 1994;154:311–6. [PubMed: 8297198](Retrospective cohort study of cases of acute liver injury in England after exposure to NSAIDs; 23 cases [none fatal] including 5 from ibuprofen, 4 diclofenac, 4 naproxen, 2 mefenamic acid, 3 ketoprofen, 2 piroxicam, 2 fenbuten and 3 sulindac; highest incidence rate [risk] for sulindac).
- Manoukian AV, Carson JL. Nonsteroidal anti-inflammatory drug-induced hepatic disorders. Incidence and prevention. Drug Saf. 1996;15:64–71. [PubMed: 8862964](Review of pharmacoepidemiology of NSAID hepatotoxicity, highlighting sulindac as having the strongest evidence of causing liver injury: 148 per 100,000 users with odds ratios for liver injury of 4.1 to 5.0).
- Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. A dynamic old drug. Clin Pharmacokinet. 1997;32:437–59. [PubMed: 9195115](Sulindac is rapidly absorbed and metabolized to sulphide which is pharmacologically active and then to a sulphone, which is inactive and excreted in the urine).
- Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1997;40:201–8. [PubMed: 9041931](Extensive review of large population based studies of NSAID liver injury, found that hepatotoxicity is rare; sulindac had highest estimated rates of 31 to 221/100,000 person-years and relative risk estimates of 2.9 to 4.1).
- Bjorkman D. Nonsteroidal anti-inflammatory drug-associated toxicity of the liver, lower gastrointestinal tract, and esophagus. Am J Med. 1998;105:17S–21S. [PubMed: 9855171](Review of gastrointestinal side effects of NSAIDs, stresses that intestinal side effects are far more common than liver).
- Tolman KG. Hepatotoxicity of non-narcotic analgesics. Am J Med. 1998;105(1B):13S–19S. [PubMed: 9715830](Review of hepatotoxicity of analgesics including NSAIDs).
- Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z, et al. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1090–7. [PubMed: 11535846](Open label study of sulindac found regional decreases in adenoma occurrence in 17 patients with familial adenomatous polyposis; no patient had side effects or serum enzyme elevations).
- O’Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. QJM. 2003;96:787–91. [PubMed: 14566034](Review of hepatotoxicity of NSAIDs stressing the increased risk from sulindac and diclofenac).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure: several were attributed to diclofenac, but none to sulindac).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; 21 drugs were associated with >50 cases, including diclofenac [15th], but not sulindac).
- Sanchez-Matienzo D, Arana A, Castellsague J, Perez-Gutthann S. Hepatic disorders in patients treated with COX-2 selective inhibitors or nonselective NSAIDs: a case/noncase analysis of spontaneous reports. Clin Ther. 2006;28:1123–32. [PubMed: 16982289](Among more than 300,000 spontaneous reports of adverse events due to NSAIDs, 3% being hepatic; the proportion of adverse events that were hepatic was highest for bromfenac [20.7%] and nimesulide [14.4%], but the proportion was also elevated for sulindac [9.9%] and diclofenac [4.7%]).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. [PubMed: 16867024](Data from Spanish and French pharmacovigilance systems from 1982-2001 found higher risk of hepatic to other types of adverse events for sulindac; 59 cases due to sulindac were listed, mostly from France).
- Aithal GP, Day CP. Nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:563–75. vi-vii. [PubMed: 17723920](Review of hepatotoxicity of NSAIDs; sulindac has been the NSAID most consistently associated with hepatotoxicity; features of hypersensitivity were present in two-thirds of cases).
- Björnsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2007;25:1411–21. [PubMed: 17539980](Analysis of case reports of drug induced liver injury for frequency and significance of eosinophilia; among 23 published cases of sulindac hepatotoxicity, the average age was 51 years, 86% were women, only 10% were hepatocellular, 12% had eosinophilia peripherally, 40% had eosinophils in liver histology, and mortality rate was 4% [1 case]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; sulindac was not listed).
- Thierman S, Dhaliwal G, Sooriash L, Baudendistel T. Flushing out the diagnosis. J Hosp Med. 2009;4:569–73. [PubMed: 20013860](42 year old woman with history of asthma developed fever, rash and jaundice 6 weeks after starting sulindac for ankle pain [bilirubin 4.6 mg/dL, ALT 667 U/L, Alk P 146 U/L, eosinophils 15%], with rapid improvement on stopping and recurrence of fever and rash on restarting twice).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, 7 of which were due to NSAIDs: 4 were linked to bromfenac, 2 diclofenac, 1 etodolac, but none to sulindac).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; sulindac is associated with a 5- to 10-fold greater risk of hepatotoxicity compared to other NSAIDs).
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, Gatta A, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf. 2013;36:135–44. [PMC free article: PMC3568201] [PubMed: 23325533](Among 600 patients undergoing liver transplantation for acute liver failure at 52 European liver transplant centers between 2005 and 2007, 301 were considered idiopathic and had received a medication within 30 days of onset, including acetaminophen in 192 and NSAIDs in 44, including diclofenac [the most commonly used NSAID] in 7; sulindac not specifically mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac [ranking 2nd], but none for sulindac or other NSAIDs).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDs [n=62, 32%], and specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW., Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016;36:603–9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most commonly diclofenac [n=16], but also celecoxib [3], meloxicam [3], etodolac [2], ibuprofen [2], oxaprozin [2], valdecoxib [1] and sulindac [1], but not naproxen).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al. DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82:238–48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] and risk was higher in those taking higher doses).
- Zoubek ME, González-Jimenez A, Medina-Cáliz I, Robles-Díaz M, Hernandez N, Romero-Gómez M, Bessone F, et al. High Prevalence of ibuprofen drug-induced Liver injury in Spanish and Latin-American registries. Clin Gastroenterol Hepatol. 2018;16:292–4. [PubMed: 28782674](Analysis of a Spanish and Latin-American registries identified 73 cases of NSAID induced liver injury, the most common agents being nimesulide [38%], diclofenac [34%] and ibuprofen [17%]; naproxen and other NSAIDs not mentioned).
- Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: challenges in diagnosis and therapy. Liver Int. 2018;38:6–14. [PMC free article: PMC5741491] [PubMed: 28771932](Review of acute liver failure and the contribution of drug induced liver injury, of which 5% were due to NSAIDs, most commonly diclofenac and etodolac).
- Meunier L, Larrey D. Recent advances in hepatotoxicity of non-steroidal anti-inflammatory drugs. Ann Hepatol. 2018;17:187–91. [PubMed: 29469052](Review of the hepatotoxicity of NSAIDS mentions the most commonly implicated are diclofenac, nimesulide, sulindac, ibuprofen, piroxicam, naproxen and aspirin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nabumetone.[LiverTox: Clinical and Researc...]Review Nabumetone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Oxaprozin.[LiverTox: Clinical and Researc...]Review Oxaprozin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Piroxicam.[LiverTox: Clinical and Researc...]Review Piroxicam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Effects of nonsteroidal antiinflammatory drugs on renal function in patients with renal insufficiency and in cirrhotics.[Am J Kidney Dis. 1986]Effects of nonsteroidal antiinflammatory drugs on renal function in patients with renal insufficiency and in cirrhotics.Brater DC, Anderson SA, Brown-Cartwright D, Toto RD. Am J Kidney Dis. 1986 Nov; 8(5):351-5.
- Sulindac-induced aseptic meningitis in mixed connective tissue disease.[Clin Neurol Neurosurg. 1989]Sulindac-induced aseptic meningitis in mixed connective tissue disease.Yasuda Y, Akiguchi I, Kameyama M. Clin Neurol Neurosurg. 1989; 91(3):257-60.
- Sulindac - LiverToxSulindac - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...