NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The triptans are a group of serotonin receptor agonists that are useful in the therapy of vascular headaches and migraine. The triptans are generally used in low doses for a limited period of time and have not been associated with serum enzyme elevations, but some have been implicated in rare instances of clinically apparent, acute cholestatic hepatitis.
Background
The triptans (trip' tans) are synthetic serotonin receptor agonists that are used in the therapy of migraine and vascular headache. Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine that has multiple actions, acting as a neurotransmitter and bioactive amine. The diversity of actions of serotonin is partially due to the multitude of different serotonin receptors and their tissue location. There are at least 15 classes of serotonin receptors which have overlapping actions, but variable distribution and intracellular pathways of response to stimulation and inhibition. The triptans are serotonin agonists with high affinity for the 5-HT1B and 5-HT1D receptors which are found on smooth-muscle cells of blood vessels. Simulation of the 5-HT1D receptor results in constriction of intracranial blood vessels. The triptans may also block the release of vasoactive peptides from perivascular trigeminal neurons through their action at presynaptic 5-HT1D receptors on nerve terminals. Regardless, the triptans have been found to be effective in preventing or aborting migraine headaches with shortening of the period of pain and symptoms. The triptans are considered “first line” agents for patients whose vascular headaches do not reliably respond to conventional analgesics. They generally have a more rapid onset of action and fewer side effects than the ergot alkaloids. Seven triptans are approved for use in the United States including almotriptan (al" moe trip' tan), eletriptan (el" e), forvatriptan (froe" va), naratriptan (nar"' a), rizatriptan (rye" za, sumatriptan (soo" ma) and zolmitriptan (zole" ma). Generic formulations are available for most agents. The short acting triptans include sumatriptan, almotriptan, eletriptan, rizatriptan and rolmitriptan and generally provide relief within 30 to 60 minutes. The longer activing oral triptans include naratriptan and frovatriptan which have a slower onset of action but may be better tolerated. Intranasal formulations may have a more rapid onset of action as do subcutaneous administered forms. Brand names, year approved, tablet or wafer size, usual dose and maximum daily recommended doses are shown in the Table.
| Generic (Brand) Name | Year Approved | Tablet (or Wafer) Size | Usual Initial Dose | Maximum 24 Hour Dose |
|---|---|---|---|---|
| Almotriptan (Axert) | 2001 | 6.25 and 12.5 mg | 12.5 mg | 25 mg |
| Eletriptan (Relpax) | 2002 | 20 and 40 mg | 40 mg | 80 mg |
| Frovatriptan (Frova) | 2001 | 2.5 mg | 2.5 mg | 7.5 mg |
| Naratriptan (Amerge) | 1998 | 1 and 2.5 mg | 2.5 mg | 5 mg |
| Rizatriptan (Maxalt) | 1998 | 5 and 10 mg | 10 mg | 30 mg |
| Sumatriptan (Imitrex) | 1997 | 25, 50 and 100 mg* | 50 mg | 200 mg |
| Zolmitriptan (Zomig) | 1997 | 2.5 mg and 5 mg** | 5 mg | 10 mg |
* Also available as nasal spray, transdermal patch and solution for injection.
** Also available in orally disintegrating tablets and as nasal spray.
Early therapy is recommended in patients with recurrent migraine, and typically the dose is repeated in 2 to 4 hours if relief has not occurred. However, the total dosage should be limited to 2 to 3 doses per 24 hour period. Parenteral and intranasal administration is helpful in patients with nausea and vomiting. Chronic, long term use of triptans to prevent migraines has been studied, but is not currently approved. The seven triptans have similar side effect profiles which include “triptan sensations” characterized by tightening of the throat, chest, neck and limbs with paresthesias and hot or cold sensations. Triptans may also cause flushing, headache, somnolence and fatigue. Rare but potentially severe adverse events include medication overuse syndrome, cerebrovascular and cardiovascular events such as myocardial infarction and stroke, serotonin syndrome and anaphylaxis.
Hepatotoxicity
In large prospective controlled trials, the different triptans have not been associated with serum enzyme elevations or hepatotoxicity; however, the frequency of monitoring in most studies was limited and rates of ALT elevations not reported. There have been rare individual reports of cholestatic hepatitis after the use of triptans, largely associated with zolmitriptan. Typically, the onset of injury was within 1 to 2 weeks of taking several doses of the zolmitriptan for a protracted and severe migraine attack. Recurrent jaundice with intermittent therapy has also been reported (Case 1). The pattern of serum enzyme elevations was mixed or cholestatic, and recovery was complete within 1 to 2 months. Allergic manifestations (rash, fever, eosinophilia) were not present and autoantibodies did not develop.
Likelihood score, zolmitriptan: D (probable rare cause of clinically apparent liver injury).
Likelihood score, rizatriptan: E* (suspected but unproven cause of liver injury).
Likelihood score, other triptans: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of idiosyncratic liver injury after triptan use is not known, but is likely due to a toxic metabolite causing an acute, cholestatic hepatitis-like injury. An intriguing hypothesis is that the serotonin agonist activity causes biliary dyskinesis and functional obstruction.
Outcome and Management
Reported cases of hepatotoxicity from the triptans have been mild-to-moderate in severity, accompanied by symptoms and jaundice, but without acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There have been too few cases reported to know whether there is cross sensitivity to hepatotoxicity among the various triptans. Rechallenge should be avoided and switching to another triptan should be done with caution.
Agents used specifically in management of migraines and vascular headaches include: the ergot alkaloids, ergotamine and dihydroergotamine; and, the serotonin receptor agonists (triptans), including almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan and zolmitriptan.
Drug Class: Migraine Headache Agents, Vasoconstrictor Agents
CASE REPORT
Case 1. Recurrent jaundice in a patient taking zolmitriptan for migraine headaches.
[Modified from: Deixler E, Helmke K. [Extrahepatic cholestasis during therapy with zolmitriptan (AscoTop)]. Z Gastroenterol 2005; 43: 1045-9. German. PubMed Citation]
A 62 year old man developed recurrent episodes of jaundice and abdominal pain approximately 6 months after starting zolmitriptan (5 mg, averaging 3-4 times weekly) for migraine headaches. During an early attack, serum bilirubin was 6.6 mg/dL (4.5 mg/dL direct), ALT 230 U/L, Alk P 350 U/L, GGT 375 U/L (Table). Tests for hepatitis A, B and C were negative as were routine autoantibodies. Because of recurrent bouts of jaundice were suggestive of cholangitis, he underwent cholecystectomy. Histologically, the gallbladder showed evidence of chronic inflammation, but was without stones and the extrahepatic biliary tree appeared normal. Three months after cholecystectomy, jaundice recurred. Endoscopic retrograde cholangiopancreatography again failed to show evidence of extrahepatic obstruction, but suggested dysfunction of the ampula of Vater. At that point, total serum bilirubin was 7.8 mg/dL, ALT 680 U/L, and alkaline phosphatase 205 U/L. Because zolmitriptan is a serotonin agonist and might affect gastrointestinal and biliary smooth muscle function, it was discontinued, whereupon liver test abnormalities rapidly improved. During a year of follow up, he had no further attacks of jaundice or abdominal pain and liver tests were repeatedly normal. His migraine headaches were managed with beta-blockers.
Key Points
| Medication: | Zolmitriptan (5 mg 3-4 times weekly) |
| Pattern: | Mixed (R=2.7) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | Six months |
| Recovery: | One month |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Zolmitriptan started for migraine headaches | |||||
| 6 months | 0 | 40 | 210 | 1.6 | Abdominal pain |
| 7 months | 0 | 30 | 200 | 1.2 | |
| 8 months | 0 | 110 | 360 | 6.6 | Cholecystectomy |
| 9 months | 0 | 90 | 200 | 1.2 | |
| 12 months | 0 | 690 | 218 | 7.8 | ERCP |
| Zolmitriptan stopped | |||||
| 13 months | 1 month | 50 | 105 | 1.1 | |
| 14 months | 2 months | 25 | 90 | 1.0 | |
| 18 months | 6 months | 25 | 70 | 1.0 | |
| Normal Values | <35 | <130 | <1.2 | ||
* Selected values estimated from Figure 1.
Comment
This patient had intermittent and fluctuating jaundice that suggested intermittent extrahepatic obstruction due to choledocholithiasis. The possibility that zolmitriptan was the cause arose only after repeated evaluations failed to show frank extrahepatic obstruction, but indicated biliary akinesia or abnormalities in the function of the ampula of Vater. The intermittency and variable doses of the migraine medication made it difficult to incriminate its use and perhaps explained the fluctuating course of liver injury. The possibility that the triptans may affect biliary kinesis is intriguing (but unproven) explanation for occasional instances of cholestatic jaundice.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Almotriptan – Generic, Almogran®, Axert®
Eletriptan – Relpax®
Frovatriptan – Frova®
Naratriptan – Generic, Amerge®
Rizatriptan – Maxalt®
Sumatriptan – Generic, Imitrex®
Zolmitriptan – Zomig®
DRUG CLASS
Migraine Headache Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Almotriptan | 154323-57-6 | C17-H25-N3-O2-S |
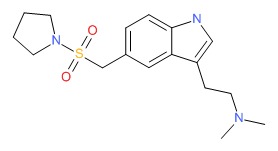 |
| Eletriptan | 177834-92-3 | C22-H26-N2-O2-S.Br-H |
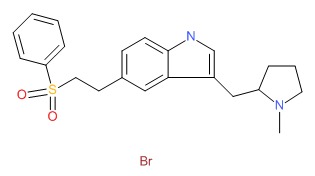 |
| Frovatriptan | 158930-17-7 | C14-H17-N3-O.C4-H6-O4.H2-O |
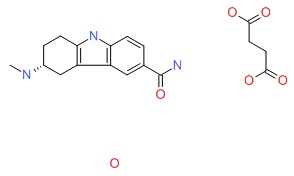 |
| Naratriptan | 121679-13-8 | C17-H25-N3-O2-S |
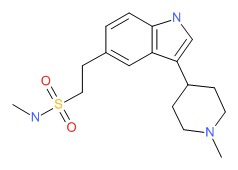 |
| Rizatriptan | 144034-80-0 | C15-H19-N5 |
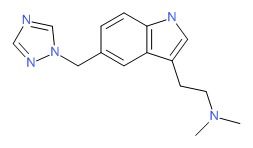 |
| Sumatriptan | 103628-46-2 | C14-H21-N3-O2-S |
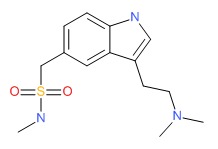 |
| Zolmitriptan | 139264-17-8 | C16-H21-N3-O2 |
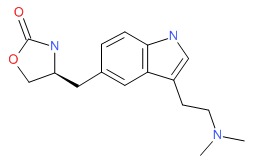 |
REFERENCES
References updated: 10 February 2018
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; ergotamine and triptans are not discussed).
- Larrey D, Ripault MP. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455. (Review of hepatotoxicity of neuroleptic drugs does not mention.ergotamine or the triptans).
- Sanders-Bush E, Mayer SE. 5-Hydroxytryptamine (Serotonin): receptor agonists and antagonists. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006, pp. 297-315.(Textbook of pharmacology and therapeutics).
- Block GA, Goldstein J, Polis A, Reines SA, Smith ME. Efficacy and safety of rizatriptan versus standard care during long-term treatment for migraine. Rizatriptan Multicenter Study Groups. Headache 1998; 38: 764-71. [PubMed: 11284464](Among 1831 patients treated for up to 12 months and experiencing 46,773 migraine attacks, pain relief was achieved in 2 hours in 80-90% on rizatriptan and 70% on standard care; side effects were similar and there were “no meaningful differences in the incidences of drug related laboratory adverse events”).
- O'Quinn S, Davis RL, Gutterman DL, Pait GD, Fox AW. Prospective large-scale study of the tolerability of subcutaneous sumatriptan injection for acute treatment of migraine. Cephalalgia 1999; 19: 223-31. [PubMed: 10376167](Among 12,339 patients in an open-label study of sumatriptan injections for migraine attacks, 4.1% had a serious adverse event, but only 20 [0.2%] were considered related to sumatriptan and none were hepatic).
- Heywood J, Bomhof MA, Pradalier A, Thaventhiran L, Winter P, Hassani H. Tolerability and efficacy of naratriptan tablets in the acute treatment of migraine attacks for 1 year. Naratriptan Long-Term Study Group. Cephalalgia 2000; 20: 470-4. [PubMed: 11037743](Among 417 patients with 15,301 migraine attacks in an open-label study of naratriptan, the most common side effects were nausea, salivation and drowsiness [2-3%] and no mention of hepatitis or liver related adverse events).
- Geraud G, Compagnon A, Rossi A; COZAM Study Group. Zolmitriptan versus a combination of acetylsalicylic acid and metoclopramide in the acute oral treatment of migraine: a double-blind, randomised, three-attack study. Eur Neurol 2002; 47: 88-98. [PubMed: 11844897](Among 666 patients with migraine treated with either zolmitriptan or aspirin/metoclopramide, side effects of paresthesias and dizziness were more common with zolmitriptan; no mention of any hepatic side effects).
- Diener HC, Jansen JP, Reches A, Pascual J, Pitei D, Steiner TJ; Eletriptan and Cafergot Comparative Study Group. Efficacy, tolerability and safety of oral eletriptan and ergotamine plus caffeine (Cafergot) in the acute treatment of migraine: a multicentre, randomised, double-blind, placebo-controlled comparison. Eur Neurol 2002; 47: 99-107. [PubMed: 11844898](Among 733 patients with migraine treated with either eletriptan or ergotamine/caffeine, side effects were transient and predominately mild or moderate; “No clinically significant laboratory…abnormalities were recorded”).
- Snow V, Weiss K, Wall EM, Mottur-Pilson C; American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med 2002; 137: 840-9. [PubMed: 12435222](Guidelines for management of acute migraine and chronic prevention; recommends use of nasal dihydroergotamine or a triptan in patients whose migraines fail to respond to aspirin, acetaminophen or nonsteroidal antiinflammatory agents).
- Jamieson DG. The safety of triptans in the treatment of patients with migraine. Am J Med 2002; 112: 135-40. [PubMed: 11835952](Review of the safety of triptans in migraine therapy focusing on vascular risk, stroke, myocardial infarction and ischemic bowel disease; no mention of hepatotoxicity).
- Redondo Cerezo E, Espinosa Aguilar MD, Nogueras López F, Martín-Vivaldi Martínez R. [Zolmitriptan-induced hepatotoxicity]. Gastroenterol Hepatol 2003; 26: 664. Spanish. [PubMed: 14670242](48 year old woman developed jaundice and itching one week after taking three doses of zolmitriptan for a severe bout of migraine [bilirubin 4.3 mg/dL, ALT 331 U/L, Alk P 530 U/L], resolving spontaneously and no rechallenge).
- Ashcroft DM, Millson D. Naratriptan for the treatment of acute migraine: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf 2004; 13: 73-82. [PubMed: 14998068](Metaanalysis of 10 trials of naratriptan for acute migraine with 4499 participants found lower rates of adverse effects with naratriptan compared to other triptans, but specifics of side effects were not provided; no mention of ALT elevations or liver injury).
- Deixler E, Helmke K. [Extrahepatic cholestasia during therapy with Zolmitriptan (AscoTop)]. Z Gastroenterol 2005; 43: 1045-9. German. [PubMed: 16142613](62 year old man developed recurrent, fluctuating jaundice and abdominal pain 6 months after starting zolmitriptan [3-4 times weekly] for migraine [bilirubin 7.8 mg/dL, ALT 690 U/L, Alk P 218 U/L], which recurred after cholecystectomy, but resolved within a month of stopping zolmitriptan: Case 1).
- Loj J, Solomon GD. Migraine prophylaxis: who, why, and how. Cleve Clin J Med 2006; 73: 793-4, 797, 800-1 passim. [PubMed: 16970133](Preventive therapy of migraine has limited efficacy and may take 2-3 months to have an effect; the most commonly used agents are beta-blockers, calcium channel blockers, anticonvulsants, tricyclic antidepressants and selective serotonin reuptake inhibitors [SSRIs]).
- Chen LC, Ashcroft DM. Meta-analysis examining the efficacy and safety of almotriptan in the acute treatment of migraine. Headache 2007; 47: 1169-77. [PubMed: 17883521](Metaanalysis of 8 controled trials of almotriptan for acute migraine with 4995 participants; no mention of ALT elevations or hepatotoxicity).
- Chen LC, Ashcroft DM. Meta-analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache 2008; 48: 236-47. [PubMed: 18179569](Metaanalysis of 24 controled trials of zolmitriptan for acute migraine with 15,408 participants; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to agents used to treat migraine headaches).
- Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother 2009; 9: 1379-91. [PubMed: 19769452](Extensive review of common side effects of medications used to treat migraine; 1-8% of patients who take triptans develop “triptan sensations with throat and chest tightness, numbness and tingling and hot/cold sensations which may vary with different forms”).
- Loder E. Triptan therapy in migraine. N Engl J Med 2010; 363: 63-70. [PubMed: 20592298](Review of the pathophysiology of migraine headache and the mechanism of action, efficacy, tolerance and safety of the serotonin receptor agonists [triptans]; no mention of ALT elevations of clinically apparent liver injury).
- Taylor FR. Acute treatment of migraine headaches. Semin Neurol 2010; 30: 145-53. [PubMed: 20352584](Review of diagnosis and acute management of migraine headaches).
- Berenson F, Vasconcellos E, Pakalnis A, Mao L, Biondi DM, Armstrong RB. Long-term, open-label safety study of oral almotriptan 12.5 mg for the acute treatment of migraine in adolescents. Headache 2010; 50: 795-807. [PubMed: 20546320](Among 319 adolescents with migraine headache treated with almotriptan for 12 months, "minor changes (increases and decreases) in mean values for hematology, serum, chemistry and urinalysis parameters were observed, but there were no consistent trends").
- Drugs for migraine. Treat Guidel Med Lett 2011; 9: 7-12. [PubMed: 21304447](Concise review of current medications used for migraine; no discussion of hepatotoxicity).
- Fernandez-Atutxa A, Vergara M, Gil M, Dalmau B, Miquel M, Sanchez-Delgado J, Casas M. [Rizatriptan-induced liver toxicity. Report of a case]. Gastroenterol Hepatol 2013; 36: 261-3. Spanish. [PubMed: 23084593](17 year old girl with migraines developed jaundice [bilirubin 7.1 mg/dL, ALT 9729 U/L, Alk P normal] two weeks after taking a 10 mg tablet of rizatriptan).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to drugs for migraine headaches).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to the triptans or other drugs for migraine headaches).
- Drugs for migraine. Med Lett Drugs Ther 2017; 59 (1514): 27-32. Corrected and republished in: JAMA 2017; 317 (21): 2230-1. PubMed Citation . [PubMed: 28170366](Concise review of current medications used for migraine; no discussion of hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Triptans: over the migraine.[Neurol Sci. 2012]Review Triptans: over the migraine.Cologno D, Mazzeo A, Lecce B, Mundi C, Petretta V, Casucci G, d'Onofrio F. Neurol Sci. 2012 May; 33 Suppl 1:S193-8.
- Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials.[Cephalalgia. 2002]Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Cephalalgia. 2002 Oct; 22(8):633-58.
- Evaluation of a monthly coverage maximum (drug-specific quantity limit) on the 5-HT1 agonists (triptans) and dihydroergotamine nasal spray.[J Manag Care Pharm. 2003]Evaluation of a monthly coverage maximum (drug-specific quantity limit) on the 5-HT1 agonists (triptans) and dihydroergotamine nasal spray.Hoffman L, Mayzell G, Pedan A, Farrell M, Gilbert T. J Manag Care Pharm. 2003 Jul-Aug; 9(4):335-45.
- Usage of triptans among migraine patients: an audit in nine GP practices.[Curr Med Res Opin. 2002]Usage of triptans among migraine patients: an audit in nine GP practices.Williams D, Cahill T, Dowson A, Fearon H, Lipscombe S, O'Sullivan E, Rees T, Strang C, Valori A, Watson D. Curr Med Res Opin. 2002; 18(1):1-9.
- Review Pharmacokinetic evaluation of frovatriptan.[Expert Opin Drug Metab Toxicol...]Review Pharmacokinetic evaluation of frovatriptan.Negro A, Lionetto L, Casolla B, Lala N, Simmaco M, Martelletti P. Expert Opin Drug Metab Toxicol. 2011 Nov; 7(11):1449-58. Epub 2011 Sep 20.
- Serotonin Receptor Agonists (Triptans) - LiverToxSerotonin Receptor Agonists (Triptans) - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...