NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The serotonin type 4 (5-HT4) receptor agonists are potent prokinetic agents that act on serotonin receptors in the intestine and promote intestinal peristalsis, increase gastric emptying and decrease esophageal reflux. Two 5-HT4 receptor agonists have been developed and were approved for use in the United States, but both were found to have significant serious adverse effects and have been withdrawn (cisapride) or placed under restricted use (tegaserod). Both cisapride and tegaserod have been associated with a low rate of transient serum enzyme elevations during therapy, but neither has been implicated convincing in cases of clinically apparent liver injury with jaundice.
Background
Serotonin plays a major role in the normal motility and secretory function of the intestine and is produced by specialized enterochromaffin cells in the mucosa of the gut. Serotonin (5-HT) is released in response to chemical and mechanical stimulation and acts through the type 4 receptors that are common in the intestinal mucosa to increase peristalsis and intestinal tone. 5-HT4 receptors are also found in the central nervous system, urinary bladder and atria of the heart, which may explain some of their adverse effects. Two 5-HT4 receptor agonists have been developed and used in clinical medicine: cisapride and tegaserod.
Cisapride (sis’ a pride) is a piperidinyl benzamide and a potent 5-HT4 receptor agonist that was developed as a therapy for gastroesophageal reflux disease (GERD) and diabetic gastroparesis. In several clinical trials, cisapride was found to improve symptoms of bloating and gastric distension in patients with gastroparesis and decrease symptoms of reflux in patients with GERD. Cisapride was approved in the United States, but was subsequently withdrawn after multiple reports of life threatening arrhythmias associated with prolonged QTc syndrome. Cisapride had been available in tablets of 10 and 20 mg under the brand name of Propulsid. The usual recommended dose was 10 to 20 mg 3 to 4 times daily. Cisapride is still available on a limited basis in Europe and elsewhere. It is also available for use in animals for treatment of gastrointestinal status and megacolon.
Tegaserod (teg” a ser’ od) is an aminoguanidine indole derivative of serotonin and a selective, partial 5-HT4 receptor agonist. It simulates the peristaltic reflex and increases intestinal and colonic motility resulting in hastened transit. It also reduces visceral sensation in response to distension. These features led to its development as therapy of irritable bowel syndrome and constipation. It was approved for use in the United States in 2002 and was available in tablets of 2 or 6 mg under the brand name of Zelnorm. The typically recommended dose was 2 to 6 mg twice daily. Tegaserod was withdrawn in 2007 because of adverse cardiovascular effects, postmarketing studies demonstrating an excess of severe adverse cardiovascular events in tegaserod treated patients (0.11%) compared to matched controls (0.01%). The association of tegaserod with adverse cardiovascular events has been contested but, currently, tegaserod is available only on an emergency basis and with prior authorization from the FDA.
Prucalopride (proo kal' oh pride) is a unique chemical entity, a benzofurancarboxamide derivative which is highly selective for the serotonin type 4 receptor and differs from cisapride and tegaserod in having minimal activity against for other serotonin receptors. In multiple clinical trials, prucalopride was found to aleviate symptoms of chronic idiopathic constipation in a proportion of patients and had minimal adverse effects. Importantly, cardiac arrhythmias and prolongation of the QTc interval were not increased in frequency in patients receiving prucalopride. Prucalopride was approved as therapy of chronic idiopathic constipation in 2018 and is now generally available. Prucalopride is described in its own record in LiverTox.
Hepatotoxicity
Both cisapride and tegaserod were linked to occasional instances of serum enzyme elevations during therapy (1% to 9%), but these elevations were generally mild and asymptomatic, resolving rapidly with or without discontinuing drug. While moderate serum enzyme elevations during 5-HT4 receptor agonist therapy have been described, there have been have been no convincing reports of clinically apparent acute liver injury with jaundice attributed to these agents. Plucalopride has not been linked to increased rates of serum enzyme elevations nor to instances of acute liver injury with jaundice, but experience with its long-term use has been limited.
Likelihood score: E (unlikely causes of clinically apparent liver injury).
Mechanism of Injury
Cisapride is metabolized in the liver, largely via CYP 3A4, and it must be used with caution in patients taking medications that inhibit CYP 3A4 activity. Tegaserod is metabolized by the liver largely by glucuronidation and has little effect on cytochrome P450 activity and few drug-drug interactions.
Drug Class: Gastrointestinal Agents, Prokinetic Agents
Other Drugs in the Subclass, Prokinetic Agents: Prucalopride
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cisapride – Generic, Propulsid®
Tegaserod – Zelnorm®
Prucalopride – Motegrity®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cisapride | 81098-60-4 | C23-H29-Cl-F-N3-O4 |
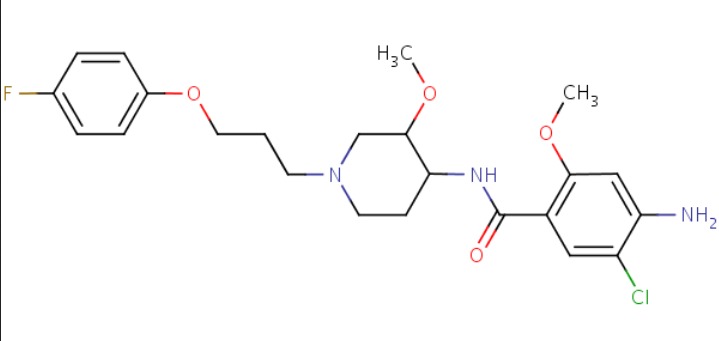 |
| Tegaserod | 145158-71-0 | C16-H23-N5-O |
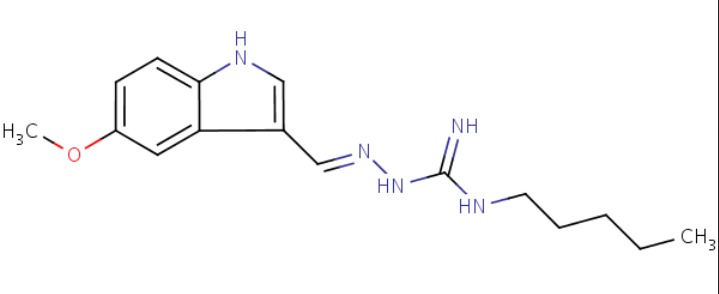 |
| Prucalopride | 179474-81-8 | C18-H26-Cl-N3-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 April 2019
- Zimmerman HJ. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 717-8.(Expert review of hepatotoxicity published in 1999 mentions that cisapride, a serotonin type 4 receptor agonist, was reported to lead to one case of liver injury [hepatocellular] during a decade of use).
- Sharkey KA,McNcaughton WK. gastointestinal motility and water flux, emesis, and biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 921-44.(Textbook of pharmacology and therapeutics; the serotonin type 4 receptor agonists are used predominantly for treatment of diseases of intestinal motility and irritable bowel syndrome).
- Havelund T, Oster-Jørgensen E, Eshøj O, Larsen ML, Lauritsen K. Effects of cisapride on gastroparesis in patients with insulin-dependent diabetes mellitus. A double-blind controlled trial. Acta Med Scand 1987; 222: 339-43. [PubMed: 3321924](Among 10 patients with diabetic gastroparesis treated in a double blind, 4 week cross over study of cisapride reported no side effects other than headache in one patient).
- Camilleri M, Malagelada JR, Abell TL, Brown ML, Hench V, Zinsmeister AR. Effect of six weeks of treatment with cisapride in gastroparesis and intestinal pseudoobstruction. Gastroenterology 1989; 96: 704-12. [PubMed: 2644150](Among 26 patients with upper gut dysmotility treated with cisapride vs placebo, symptoms improved equally in both groups, although measured gastric emptying changed only with cisapride; side effects were not mentioned).
- Galmiche JP, Fraitag B, Filoche B, Evreux M, Vitaux J, Zeitoun P, Fournet J, Soule JC. Double-blind comparison of cisapride and cimetidine in treatment of reflux esophagitis. Dig Dis Sci 1990; 35: 649-55. [PubMed: 2331957](Among 73 patients with erosive reflux esophagitis treated with cisapride or cimetidine, symptoms and mucosal healing occurred equally with either drug and side effects were mild; no mention of ALT levels or hepatotoxicity).
- Abell TL, Camilleri M, DiMagno EP, Hench VS, Zinsmeister AR, Malagelada JR. Long-term efficacy of oral cisapride in symptomatic upper gut dysmotility. Dig Dis Sci 1991; 36: 616-20. [PubMed: 2022163](Among 21 patients with gastroparesis treated with cisapride for 12 months, "no significant side effects were noted").
- Denié C, Gohy P. [Cytolytic hepatitis induced by cisapride]. Gastroenterol Clin Biol 1992; 16: 368-9. French. [PubMed: 1397860](A 67 year old man with GERD developed nausea 4 days after starting cisapride, and one week later was still symptomatic and was found to have abnormal liver tests [bilirubin 1.2 mg/dL, ALT 11 times ULN, Alk P 1.2 times ULN, normal amylase], abnormalities resolving within 3 weeks of stopping).
- Richards RD, Valenzuela GA, Davenport KG, Fisher KL, McCallum RW. Objective and subjective results of a randomized, double-blind, placebo-controlled trial using cisapride to treat gastroparesis. Dig Dis Sci 1993; 38: 811-6. [PubMed: 8482178](Among 38 patients with gastroparesis treated with cisapride or placebo for 6 weeks, there was "no significant change in any of the measured laboratory parameters relative to baseline").
- Dworkin BM, Rosenthal WS, Casellas AR, Girolomo R, Lebovics E, Freeman S, Clark SB. Open label study of long-term effectiveness of cisapride in patients with idiopathic gastroparesis. Dig Dis Sci 1994; 39: 1395-8. [PubMed: 8026248](In an open label study in 11 patients with idiopathic gastroparesis treated with cisapride for 12 months, "no significant side effects of cisapride, either in clinical or laboratory data, were observed").
- Kellow JE, Cowan H, Shuter B, Riley JW, Lunzer MR, Eckstein RP, Höschl R, et al. Efficacy of cisapride therapy in functional dyspepsia. Aliment Pharmacol Ther 1995; 9: 153-60. [PubMed: 7605855](Among 74 patients treated with cisapride or placebo for 4 weeks, adverse events were "generally mild" and hepatotoxicity and ALT levels were not mentioned).
- Richter JE, Long JF. Cisapride for gastroesophageal reflux disease: a placebo-controlled, double-blind study. Am J Gastroenterol 1995; 90: 423-30. [PubMed: 7872282](Among 177 patients with GERD treated with cisapride or placebo for 2 weeks, adverse events included diarrhea and constipation, and "no significant changes occurred in values for...clinical laboratory variables").
- Bashir RM, Lewis JH. Hepatotoxicity of drugs used in the treatment of gastrointestinal disorders. Gastroenterol Clin North Am 1995; 24: 937-67. [PubMed: 8749906](Review of evidence of hepatotoxicity of gastrointestinal drugs includes mention that cisapride showed no evidence of hepatotoxicity in prelicensure studies and that only a single case of hepatotoxicity attributed to cisapride has been published [Denié 1992]).
- Castell DO, Sigmund C Jr, Patterson D, Lambert R, Hasner D, Clyde C, Zeldis JB. Cisapride 20 mg b.i.d. provides symptomatic relief of heartburn and related symptoms of chronic mild to moderate gastroesophageal reflux disease. CIS-USA-52 Investigator Group. Am J Gastroenterol 1998; 93: 547-52. [PubMed: 9576446](Among 398 patients with symptomatic GERD treated with cisapride or placebo for 4 weeks, diarrhea was more common with cisapride [10% vs 4%]; laboratory testing results were not mentioned).
- Castell D, Silvers D, Littlejohn T, Orr W, Napolitano J, Oleka N, Jokubaitis L. Cisapride 20 mg b.d. for preventing symptoms of GERD induced by a provocative meal. The CIS-USA-89 Study Group. Aliment Pharmacol Ther 1999; 13: 787-94. [PubMed: 10383509](Among 122 patients treated with cisapride or placebo for 7 days, diarrhea occurred in 18% of cisapride vs 0% of placebo recipients; laboratory measurements were not done).
- Tougas G, Snape WJ Jr, Otten MH, Earnest DL, Langaker KE, Pruitt RE, Pecher E, et al. Long-term safety of tegaserod in patients with constipation-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2002; 16: 1701-8. [PubMed: 12269961](Among 567 patients with constipation predominant IBS treated with tegaserod for up to 12 months, common adverse events were diarrhea [10%], headache [8%], and abdominal pain [7%], but blood tests results were "generally unremarkable" and no serious hepatic adverse events occurred).
- Hasler WL, Schoenfeld P. Safety profile of tegaserod, a 5-HT4 receptor agonist, for the treatment of irritable bowel syndrome. Drug Saf 2004; 27: 619-31. [PubMed: 15230644](Review of the safety of tegaserod in IBS mentions that laboratory parameters were "mostly unaffected by tegaserod").
- Nyhlin H, Bang C, Elsborg L, Silvennoinen J, Holme I, Rüegg P, Jones J, et al. A double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety and tolerability of tegaserod in patients with irritable bowel syndrome. Scand J Gastroenterol 2004; 39: 119-26. [PubMed: 15000272](Among 643 patients with IBS treated with tegaserod [6 mg twice daily] or placebo for 12 weeks, diarrhea [9.2% vs 1.3%] and headache [8% vs 4.7%] were more frequent with tegaserod, but laboratory data .did not raise any issues.).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to prokinetic agents or the 5-HT4 receptor agonists).
- Müller-Lissner S, Kamm MA, Musoglu A, Earnest DL, Dunger-Baldauf C, Shetzline MA. Safety, tolerability, and efficacy of tegaserod over 13 months in patients with chronic constipation. Am J Gastroenterol 2006; 101: 2558-69. [PubMed: 17090282](Among 842 patients with chronic constipation treated in an open label extension study with tegaserod [2 or 6 mg twice daily] for up to 13 months, side effects included headache, abdominal pain, diarrhea, nausea, and ALT elevations which occurred in 3.7% receiving 4 mg and 6.8-9.7% receiving 12 mg daily).
- Quigley EM, Wald A, Fidelholtz J, Boivin M, Pecher E, Earnest D. Safety and tolerability of tegaserod in patients with chronic constipation: pooled data from two phase III studies. Clin Gastroenterol Hepatol 2006; 4: 605-13. [PubMed: 16678076](In a pooled analysis of 2 controlled trials of 12 weeks of tegaserod vs placebo, clinical chemistry results "were generally comparable across all treatment groups with no clinically significant trends observed").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to prokinetic agents or the 5-HT4 receptor agonists).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to prokinetic agents or the 5-HT4 receptor agonists).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, 1% were hepatic, but no prokinetic agent or 5-HT4 receptor agonist was listed among the 41 most commonly implicated agents).
- Chey WD, Howden CW, Tack J, Ligozio G, Earnest DL. Long-term tegaserod treatment for dysmotility-like functional dyspepsia: results of two identical 1-year cohort studies. Dig Dis Sci 2010; 55: 684-97. [PubMed: 19957035](Among 780 patients with IBS treated with tegaserod for up to 1 year, most adverse events occurred in the first 6 months and "there were no clinically relevant changes in mean values" of routine biochemical results).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 114: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to prokinetic agents or the 5-HT4 receptor agonists).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common implicated agents being nimesulide [n=53: 30%], cyproterone [n=18], nitrofurantoin [n=17], antituberculosis drugs [n=13], and flutamide [n=12: 7%]; prokinetic agents or the 5-HT4 receptor agonists were not listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to metoclopramide, but none to cisapride or other prokinetic agents).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Measurement of 5-HT4 receptor-mediated esophageal responses by digital sonomicrometry in the anesthetized rat.[J Pharmacol Toxicol Methods. 2...]Measurement of 5-HT4 receptor-mediated esophageal responses by digital sonomicrometry in the anesthetized rat.Armstrong SR, McCullough JL, Beattie DT. J Pharmacol Toxicol Methods. 2006 May-Jun; 53(3):198-205. Epub 2005 Sep 15.
- Inotropic effects of prokinetic agents with 5-HT(4) receptor agonist actions on human isolated myocardial trabeculae.[Life Sci. 2012]Inotropic effects of prokinetic agents with 5-HT(4) receptor agonist actions on human isolated myocardial trabeculae.Chai W, Chan KY, de Vries R, van den Bogeardt AJ, de Maeyer JH, Schuurkes JA, Villalón CM, Saxena PR, Danser AH, MaassenVanDenBrink A. Life Sci. 2012 Apr 9; 90(13-14):538-44. Epub 2012 Feb 1.
- Review Irritable bowel syndrome: new agents targeting serotonin receptor subtypes.[Drugs. 2001]Review Irritable bowel syndrome: new agents targeting serotonin receptor subtypes.De Ponti F, Tonini M. Drugs. 2001; 61(3):317-32.
- The in vitro pharmacology and non-clinical cardiovascular safety studies of a novel 5-HT(4) receptor agonist, DSP-6952.[Eur J Pharmacol. 2018]The in vitro pharmacology and non-clinical cardiovascular safety studies of a novel 5-HT(4) receptor agonist, DSP-6952.Tsubouchi T, Kunimatsu T, Tsujimoto S, Kiyoshi A, Katsura Y, Oku S, Chihara K, Mine Y, Yamada T, Shimizu I, et al. Eur J Pharmacol. 2018 May 5; 826:96-105. Epub 2018 Mar 1.
- Review Prucalopride.[LiverTox: Clinical and Researc...]Review Prucalopride.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Serotonin 5-HT4 Receptor Agonists - LiverToxSerotonin 5-HT4 Receptor Agonists - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...