NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Procainamide is an oral antiarrhythmic agent that has been in use for more than 60 years. Long term procainamide therapy is known to induce hypersensitivity reactions, autoantibody formation and a lupus-like syndrome but is a rare cause of clinically apparent acute liver injury.
Background
Procainamide (proe kane' a mide) is an analogue of the local anesthetic procaine and has electrophysiological effects that resemble quinidine. Procainamide appears to act by blocking open sodium channels and outward potassium channels. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction. Procainamide was approved for use in the United States in 1950, and current indications include suppression of symptomatic premature ventricular contractions and life threatening ventricular tachycardia, as well as maintenance of normal sinus rhythm after conversion of atrial fibrillation or flutter. Because of its safety profile, procainamide is now rarely used and is recommended only for life-threatening ventricular arrhythmias. Procainamide was formerly available in capsules and tablets of 250, 375 and 500 mg generically and under the brand name Pronestyl and as extended release forms of 250, 500, 750 and 1,000 mg under the brand name Procanbid. Procainamide is currently available as a solution for intravenous infusion. The usual maintenance oral dose in adults is 500 to 1000 mg every 4 to 6 hours. The most common side effects include headache, nervousness, anxiety, nausea, decreased appetite, palpitations and disturbed sleep. Rare but potentially severe adverse events include aplastic anemia, agranulocytosis, drug hypersensitivity reactions, induction of autoantibodies including antinuclear antibody and drug induced lupus erythematosus.
Hepatotoxicity
In clinical trials, procainamide was associated with a low rate of serum aminotransferase and alkaline phosphatase elevations. Despite wide scale use, procainamide has only rarely been linked to cases of clinically apparent liver injury. In reported cases, fever and mild symptoms arose within 1 to 3 weeks of starting (or within 1 day of restarting) procainamide, associated with a cholestatic pattern of serum enzyme elevations with mild or no jaundice (Case 1). Immunoallergic features were usually present (fever, rash, leukocytosis). In reported cases, fever resolved immediately and evidence of liver injury within a few days to weeks of stopping procainamide. Liver biopsy may how granulomas in addition to mild nonspecific changes. Interestingly, the hepatotoxicity of procainamide closely resembles that of quinidine, but there is no apparent cross sensitivity to the hepatic injury. In addition, up to 20% of patients on long term procainamide therapy develop autoantibodies, including ANA and LE prep positivity and a proportion develop a “lupus-like” syndrome. These autoimmune conditions, however, typically occur without an accompanying hepatitis, serum enzyme elevations or jaundice.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which procainamide causes liver injury is likely hypersensitivity. Procainamide is extensively metabolized in the liver which might result in production of an immunogenic metabolite.
Outcome and Management
The liver injury due to procainamide is usually a part of a hypersensitivity reaction with fever, rash and fatigue and resolves rapidly with discontinuation. Rechallenge invariably leads to recurrence of the fever and liver test abnormalities and should be avoided. There does not appear to be cross sensitivity between hypersensitivity reactions to procainamide and those due to quinidine or other antiarrhythmics.
Drug Class: Antiarrhythmic Agents
CASE REPORT
Case 1. Acute hypersensitivity and liver injury after an infusion of procainamide.(1)
A 23 year old man with Wolff-Parkinson-White syndrome developed a fever and rash 6 hours after an infusion of procainamide (1.5 g intravenously), given as a part of a cardiac electrophysiological study. He had received procainamide three weeks previously to reverse a supraventricular tachycardia that was thereafter managed with oral nadolol (40 mg daily) until the planned cardiac study. He had no history of liver disease, alcohol abuse, risk factors for viral hepatitis or previous drug allergies. He took no other medications. On examination, he was febrile to 38.9 oC and had a diffuse macular erythematous rash. There was slight tenderness over his liver. Laboratory testing showed a slight elevation in white blood cell count and elevations in liver tests (Table). Tests for hepatitis A and B were negative as were autoantibodies. He was monitored on no therapy and his symptoms resolved rapidly. The liver test abnormalities worsened for a few days and then began to improve.
Key Points
| Medication: | Procainamide (1500 mg intravenously) |
|---|---|
| Pattern: | Mixed (R=2.9) |
| Severity: | 3+ (jaundice, prolongation of hospitalization) |
| Latency: | 6 hours |
| Recovery: | Not given |
| Other medications: | Nadolol |
Laboratory Values
Comment
This patient developed a hypersensitivity reaction to a single infusion of procainamide (a second exposure) with fever, rash and leukocytosis (eosinophils count was not given), followed by jaundice and a mixed pattern of serum enzyme elevations. Most instances of procainamide associated liver injury have been associated with fever and other manifestations of hypersensitivity. The pattern of liver enzyme elevations is usually cholestatic and the injury usually self-limiting and mild.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Procainamide – Generic, Pronestyl®
DRUG CLASS
Antiarrhythmic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Procainamide | 51-06-9 | C13-H21-N3-O |
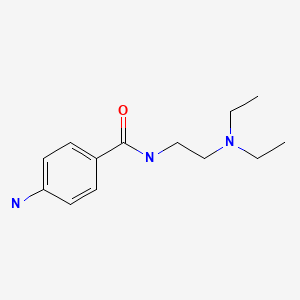
|
CITED REFERENCE
- 1.
- Worman HJ, Ip JH, Winters SL, Tepper DC, Gomes AJ. Hypersensitivity reaction associated with acute hepatic dysfunction following a single intravenous dose of procainamide. J Intern Med. 1992;232:361–3. [PubMed: 1402640]
ANNOTATED BIBLIOGRAPHY
References updated: 10 July 2020
Abbreviations: ANA, antinuclear antibody; LE prep, Lupus erythematosus preparation.
- Zimmerman HJ. Antiarrhythmics. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity of antiarrhythmics published in 1999; “Hepatic injury caused by this drug appears to be rare” 6 cases having been reported).
- De Maurzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of antiarrhythmics mentions that liver injury from procainamide is rare and generally mild with features of hypersensitivity).
- Knollman BC, Roden DM. Anti-arrhythmic drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 547-72.(Textbook of pharmacology and therapeutics).
- Bakos AC, Askey JM. Fever due to procaine amide hydrochloride therapy. J Am Med Assoc. 1952;149:1393. [PubMed: 14938192](58 year old woman developed fever and nausea 11 days after starting procainamide, resolving promptly on stopping and recurring after a single dose; liver tests were not done).
- Hellman E. Allergy to procaine amide. J Am Med Assoc. 1952;149:1393–4. [PubMed: 14938193](53 year old man developed rash within a day of starting procainamide followed by fever, adenopathy and eosinophilia; liver tests not done).
- McGarry JJ. Chills and fever due to procaine amide hydrochloride therapy. N Engl J Med. 1952;247:1033–4. [PubMed: 13002668](57 year old man developed recurrent fevers within one week of starting procainamide, resolving rapidly with stopping and recurring within 24 hours of rechallenge; no mention of liver tests or jaundice; negative patch and skin tests).
- Luton EF. Febrile reaction to procainamide therapy. J Am Med Assoc. 1959;170:43–5. [PubMed: 13640982](61 year old man developed fever within a day of starting procainamide followed by fatigue, resolving within 24 hours of stopping with 3 subsequent positive rechallenges [fever]; no mention of liver tests).
- Ladd AT. Procainamide-induced lupus erythematosus. N Engl J Med. 1962;267:1357–8. [PubMed: 13927984](49 year old man developed intermittent rash and then arthralgias 4 months after starting procainamide with low grade fever, pleurisy, lupus erythematosus prep and ANA positivity, resolving with stopping and recurrence of fever [without LE prep positivity] 3 days after restarting procainamide).
- King JA. Blountre Jr. An unexpected reaction to procainamide. JAMA. 1963;186:603–4. [PubMed: 14059044](78 year old woman with recurrent bouts of fever and painful hepatomegaly occurring after taking procainamide with two positive rechallenges, ALT rising to 85-185 U/L during episodes, but normal bilirubin and Alk P).
- Colman RW, Sturgill BC. Lupus-like syndrome induced by procainamide: association with anti-DNA antibody. Arch Intern Med. 1965;115:214–6. [PubMed: 14332007](40 year old woman developed arthritis 4 months after starting procainamide; at 6 months ANA 1:4096, positive LE cell preps [AST and bilirubin normal]; symptoms and ANA had resolved 7 months after stopping).
- Kaplan JM, Wachtel HL, Czarnecki SW, Sampson JJ. Lupus-like illness precipitated by procainamide hydrochloride. JAMA. 1965;192:444–7. [PubMed: 14284842](4 female patients, ages 47-70 years, developed arthralgias after 4-20 weeks of procainamide therapy with pleurisy, LE prep and ANA positivity, normal ALT and bilirubin, resolving in 8-24 weeks, rapid recurrence upon rechallenge).
- Rosin JM. Vasculitis following procaine amide therapy. Am J Med. 1967;42:625–9. [PubMed: 6023008](53 year old man with atherosclerosis developed cyanosis and vasculitis in both hands 4 weeks after starting procainamide; positive LE prep resolved upon stopping, but fingers were subsequently lost from gangrene).
- Lappat EJ, Cawein MJ. A familial study of procainamide-induced systemic lupus erythematosus. A question of pharmacogenetic polymorphism. Am J Med. 1968;45:846–52. [PubMed: 4177546](51 year old woman developed arthritis 5 months after starting procainamide [LE prep and ANA positive], resolving upon stopping, patients mother and 1 of 3 children were ANA positive and had nuclear phagocytosis on LE preps).
- Dubois EL. Procainamide induction of a systemic lupus erythematosus-like syndrome. Presentation of six cases, review of the literature, and analysis and followup of reported cases. Medicine (Baltimore). 1969 May;48:217–28. [PubMed: 5769742](Extensive review of literature [55 cases] and description of 6 new cases, 6 patients with lupus-like syndrome after procainamide therapy; 5 men and 1 women, ages 7 to 77 years, treated for 1-72 months [mean=5], arthralgias in 92%, fever 39%, LE prep present 94%; skin rash less common than with idiopathic lupus and renal involvement very rare even on autopsy).
- Molina J, Dubois EL, Bilitch M, Bland SL, Friou GJ. Procainamide-induced serologic changes in asymptomatic patients. Arthritis Rheum. 1969;12:608–14. [PubMed: 4188607](Among 22 patients treated with procainamide for >6 weeks, 17 [77%] developed LE prep positivity and 2 [10%] lupus-like syndrome).
- Drug-induced lupus syndromes. Br Med J. 1970;2:192–3. [PMC free article: PMC1700051] [PubMed: 5443401](Editorial on drug induced lupus syndromes focusing upon hydralazine, procainamide, isoniazid, and anticonvulsants).
- Blomgren SE. Drug-induced lupus erythematosus. Semin Hematol. 1973;10:345–9. [PubMed: 4803375](Review of drugs that cause a lupus-like syndrome, focusing upon hydralazine and procainamide in whom >10% developed lupus and >50% ANA positivity; syndrome also reported with isoniazid, penicillin, anticonvulsants and chlorpromazine).
- Farber HI. Fever, vomiting, and liver dysfunction with procainamide therapy. Postgrad Med. 1974;56:155–6. [PubMed: 4835003](58 year old developed fever 3 weeks after starting procainamide [ALT 140 U/L, Alk P 14.4 BU, and bilirubin 2.0 mg/dL], resolving rapidly on stopping drug).
- Henningsen NC, Cederberg A, Hanson A, Johansson BW. Effects of long-term treatment with procaine amide. A prospective study with special regard to ANF and SLE in fast and slow acetylators. Acta Med Scand. 1975;198:475–82. [PubMed: 55060](Among 42 patients treated with procainamide for >3 months, 35 [83%] developed ANA positivity and 12 [29%] a lupus-like syndrome; more likely to occur in slow acetylators).
- Woosley RL, Drayer DE, Reidenberg MM, Nies AS, Carr K, Oates JA. Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med. 1978;298:1157–9. [PubMed: 306574](Among 20 patients treated with procainamide chronically, 18 developed ANA positivity [90%], but time to positivity was sooner among slow acetylators [50% positive by 2.9 vs 7.3 months]).
- Rotmensch HH, Yust I, Siegman-Igra Y, Liron M, Ilie B, Vardinon N. Granulomatous hepatitis: a hypersensitivity response to procainamide. Ann Intern Med. 1978;89:646–7. [PubMed: 717937](63 year old man developed fatigue and fever 2 weeks after starting procainamide followed by pruritus [bilirubin 2.0 mg/dL, Alk P 5 times ULN, ALT 130 U/L, LE prep and ANA negative], resolving rapidly on stopping; biopsy showed granulomas, positive rechallenge with fever and ALT elevation [62 U/L]).
- Bernstein RE. Procainamide, acetylprocainamide, and drug-induced lupus erythematosus. Lancet. 1979;2:1076. [PubMed: 91813](Letter arguing that autoantibody induction by procainamide therapy is due to its slow metabolism by N-acetyltransferase and that acetylprocainamide would be better tolerated).
- Rotmensch HH, Weintraub M, Sofferman G, Livni E, Klejman A, Liron M. Experience with immunological tests in drug-induced hepatitis. Z Gastroenterol. 1981;19:691–7. [PubMed: 7032097](Experience in using mast cell degranulation tests to validate causality of drug induced liver disease, including patient with procainamide induced liver injury: same case as in Rotmensch 1978).
- McMaster KR 3rd, Hennigar GR. Drug-induced granulomatous hepatitis. Lab Invest. 1981;44:61–73. [PubMed: 7453131](Analysis of 95 cases of granulomatous hepatitis [6% of liver biopsies]; 29% due to drugs including 1 attributed to procainamide arising within 1 week and with fever but no rash, eosinophilia nor ANA reactivity).
- Kumar KL, Reuler JB. Drug fever. West J Med. 1986;144:753–5. [PMC free article: PMC1306783] [PubMed: 3487884](Review of the causes and forms of drug fever; most common is hypersensitivity reactions such as occurs with procainamide).
- Knox JP, Welykyj SE, Gradini R, Massa MC. Procainamide-induced urticarialvasculitis. Cutis. 1988;42:469–72. [PubMed: 2973973](Two male patients, aged 70 and 74 years, developed urticaria and vasculitis 7 months and 3 years after starting procainamide with ANA positivity, resolving 3-4 days after stopping procainamide; liver tests normal).
- Ahn CS, Tow DE. Intrahepatic cholestasis due to hypersensitivity reaction to procainamide. Arch Intern Med. 1990;150:2589–90. [PubMed: 2244779](71 year old man developed fever 4 days after starting procainamide with rise in serum bilirubin [0.3 to 13.0 mg/dL], ALT [14 to 63 U/L] and Alk P [92 to 734 U/L], jaundice persisting for more than 2 weeks, resolution not documented).
- Worman HJ, Ip JH, Winters SL, Tepper DC, Gomes AJ. Hypersensitivity reaction associated with acute hepatic dysfunction following a single intravenous dose of procainamide. J Intern Med. 1992;232:361–3. [PubMed: 1402640](23 year old man developed fever and rash 4 hours after an infusion of procainamide [second exposure] and in next few days bilirubin rose from 0.3 to 6.5 mg/dL, ALT 46 to 394 U/L, Alk P 73 to 252 U/L, resolving rapidly thereafter: Case 1).
- Chuang LC, Tunier AP, Akhtar N, Levine SM. Possible case of procainamide-induced intrahepatic cholestatic jaundice. Ann Pharmacother. 1993;27:434–7. [PubMed: 8477118](77 year old woman became obtunded 6 weeks after starting procainamide [bilirubin 5.2 rising to 10.1 mg/dL, ALT 382 U/L, Alk P 1071 U/L, ANA 1:40], macular rash, improving upon stopping procainamide and with prednisone therapy, but incomplete follow up).
- Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135–47. [PubMed: 15767026](More than 40 medications have been linked to drug induced lupus; up to 20% of patients on long term procainamide will develop lupus-like syndrome; animal models have used a hydroxylamine derivative of procainamide which may act as an immunogenic hapten).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, no cases were attributed to procainamide).
- Drugs for cardiac arrhythmias. Treat Guidel Med Lett. 2007;5:51–8. [PubMed: 17505408](Concise review of drugs for arrhythmias; procainamide is considered a second line, or alternative choice for both ventricular and atrial arrhythmias; side effects are common and can include lupus-like syndrome, confusion, dyspepsia, rash, hypotension and blood dyscrasias).
- Treatment of atrial fibrillation. Treat Guidel Med Lett. 2010;8(97):65–70. [PubMed: 20733547](Concise review of efficacy and safety of drugs for atrial fibrillation; first line agents include amiodarone, dronedarone, propafenone and flecainide; procainamide can prolong the QTc interval and is not recommended).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to procainamide).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 cases [0.8%] were attributed to antiarrhythmics, [5 to amiodarone, 2 to dronedarone], but none to procainamide).
- Arnaud L, Mertz P, Gavand PE, Martin T, Chasset F, Tebacher-Alt M, Lambert A, et al. Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann Rheum Dis. 2019;78:504–8. [PubMed: 30793701](Among 8136 reports of drug induced systemic lupus reported to the VigilBase registry of drug adverse events between 1967 and 2018, 118 suspect drugs were identified the most frequent being infliximab [1053], adalimumab [926], etanercept [691], procainamide [518: 6%] and hydralazine [444: 5.1%]; 81% of cases in women, median age 49 years, median onset 172 days).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide.[Drug Saf. 1990]Review Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide.Kim SY, Benowitz NL. Drug Saf. 1990 Nov-Dec; 5(6):393-420.
- Hypersensitivity reaction associated with acute hepatic dysfunction following a single intravenous dose of procainamide.[J Intern Med. 1992]Hypersensitivity reaction associated with acute hepatic dysfunction following a single intravenous dose of procainamide.Worman HJ, Ip JH, Winters SL, Tepper DC, Gomes AJ. J Intern Med. 1992 Oct; 232(4):361-3.
- Antiarrhythmic efficacy, pharmacokinetics and safety of N-acetylprocainamide in human subjects: comparison with procainamide.[Am J Cardiol. 1980]Antiarrhythmic efficacy, pharmacokinetics and safety of N-acetylprocainamide in human subjects: comparison with procainamide.Roden DM, Reele SB, Higgins SB, Wilkinson GR, Smith RF, Oates JA, Woosley RL. Am J Cardiol. 1980 Sep; 46(3):463-8.
- Procainamide, N-acetylprocainamide, antinuclear antibody and systemic lupus erythematosus.[Angiology. 1986]Procainamide, N-acetylprocainamide, antinuclear antibody and systemic lupus erythematosus.Reidenberg MM, Drayer DE. Angiology. 1986 Dec; 37(12 Pt 2):968-71.
- Review Procainamide: a perspective on its value and danger.[Heart Dis Stroke. 1993]Review Procainamide: a perspective on its value and danger.Ellenbogen KA, Wood MA, Stambler BS. Heart Dis Stroke. 1993 Nov-Dec; 2(6):473-6.
- Procainamide - LiverToxProcainamide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...