NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Procarbazine is an orally administered alkylating agent used in combination with other antineoplastic agents in the therapy of Hodgkin’s disease and malignant melanoma. Procarbazine therapy has been associated with serum enzyme elevations during therapy and with rare cases of idiosyncratic, clinically apparent acute liver injury.
Background
Procarbazine (proe kar' ba zeen) is a methylhydrazine derivative which is activated in the liver to highly reactive alkylating intermediates. These intermediates methylate DNA which causes inhibition of DNA, RNA and protein synthesis and cell death. Procarbazine was approved for use in the United States in 1969 and it remains a commonly used agent in the treatment of Hodgkin’s and non-Hodgkin’s lymphomas and brain cancer. Procarbazine is rarely used alone, but is found in common cancer chemotherapeutic regimens such as MOPP (mechlorethamine, vincristine [oncovin], procarbazine and prednisone), COPP (cyclophosphamide, vincristine [oncovin], procarbazine and prednisone), and PCV (procarbazine, lomustine [CCNU], vincristine). Procarbazine is available as tablets of 50 mg generically and under the brand name Matulane. It is typically given in monthly or every other month cycles of 10 to 14 days in a dose of 100 mg per meter squared body surface area. Common side effects are alopecia, anoxia, nausea, vomiting, headache, peripheral neuropathy, and flu-like illness. Severe adverse events include myelosuppression, neurologic and renal dysfunction.
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels are not uncommon during courses of systemic combination chemotherapy and the role of procarbazine in these abnormalities is often not clear. Aminotransferase elevations arise in more than half of patients and rise above 5 times ULN in 10 to 20% of patients. However, dose modification for serum enzyme elevations is rarely necessary. Clinically apparent liver disease with fever and marked elevations in serum aminotransferase levels without jaundice has been reported but is very rare. A single case report described self-limited, hepatocellular injury without jaundice during a second course of combination therapy and recurrence upon rechallenge with procarbazine, but not with the other antineoplastic agents being used.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of hepatotoxicity from procarbazine is not known, but may be due to hypersensitivity.
Outcome and Management
The severity of liver injury from procarbazine is usually mild and self-limiting. Procarbazine therapy has not been associated with cases of acute liver failure, chronic liver injury or vanishing bile duct syndrome. The product label for procarbazine recommends obtaining routine liver and kidney tests before starting and every week during therapy. Elevations of aminotransferase levels above 5 times ULN should be managed by temporary discontinuation of procarbazine until the abnormalities resolve.
Drug Class: Antineoplastic Agents, Alkylating Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Procarbazine – Matulane®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
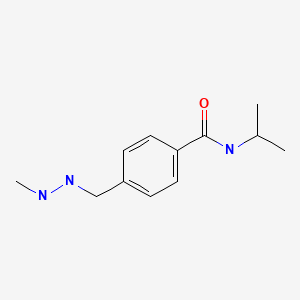
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Procarbazine | 671-16-9 | C12-H19-N3-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 September 2020
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that procarbazine appears to produce little hepatic injury, and its other side effects outweigh the importance of its hepatotoxicity).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents does not discuss procarbazine).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-201.(Textbook of pharmacology and therapeutics).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. [PubMed: 3321587](Among 235 patients undergoing bone marrow transplantation between 1982 and 1985, sinusoidal obstruction syndrome [SOS] developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Fesler MJ, Becker-Koepke S, Di Bisceglie AM, Petruska PJ. Procarbazine-induced hepatotoxicity: case report and review of the literature. Pharmacotherapy. 2010;30:540. [PubMed: 20412004](65 year old man with lymphoma developed fever and transient marked elevations in ALT [~2100 U/L] and LDH [873 U/L] during second course of C-MOPP-R and had a positive rechallenge to procarbazine [fever and ALT elevations to ~800 U/L], but no recurrence with other components of the treatment regimen).
- Hallén K, Sangfelt P, Nilsson T, Nordgren H, Wanders A, Molin D. Vanishing bile duct-like syndrome in a patient with Hodgkin lymphoma – pathological development and restitution. Acta Oncol. 2014;53:1271–5. [PubMed: 24697745](Man of unstated age developed jaundice and severe pruritus with vanishing bile duct syndrome due to Hodgkin disease [bilirubin 5.5 rising to 34 mg/dL], eventually responding after courses of procarbazine containing combination chemotherapies and having normal liver tests in long term follow up).
- Thakar K, Novero A, Das A, Lisinschi A, Mehta B, Ahmed T, Liu D. CEPP regimen (cyclophosphamide, etoposide, procarbazine and prednisone) as initial treatment for Hodgkin lymphoma patients presenting with severe abnormal liver function. Biomark Res. 2014;2:12. [PMC free article: PMC4078319] [PubMed: 24991411](Two patients with Hodgkin disease presented with jaundice and responded to chemotherapy with cyclophosphamide, etoposide, procarbazine and prednisone with improvement in the liver disease allowing for more aggressive chemotherapy).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 363 [36%] were attributed to antibiotics, none of which was attributed to procarbazine).
- Jutras G, Bélanger K, Letarte N, Adam JP, Roberge D, Lemieux B, Lemieux-Blanchard É, et al. Procarbazine, lomustine and vincristine toxicity in low-grade gliomas. Curr Oncol. 2018;25:e33–e39. [PMC free article: PMC5832288] [PubMed: 29507493](Among 57 patients with low grade gliomas treated with procarbazine, lomustine and vincristine at a single center between 2005 and 2015, hematologic, hepatologic and other toxicities were common, ALT elevations occurring in 65% and rising to above 5 times ULN in 18% of patients, usually attributed to and leading to discontinuation of procarbazine, but not resulting in any toxic deaths).
- Fong M, Boyle S, Gutta N. Brentuximab vedotin in combination with sequential procarbazine, cyclophosphamide and prednisolone for the management of Hodgkin's lymphoma-associated vanishing bile duct syndrome (VBDS) with severe obstructive liver failure. BMJ Case Rep. 2019;12:e227676. [PMC free article: PMC6388893] [PubMed: 30796069](39 year old woman with jaundice, severe itching and vanishing bile duct syndrome due to Hodgkin disease [bilirubin 9.9 rising to 23.6 mg/dL, ALT 8 times and Alk P 5 times ULN] responded to therapy with cyclophosphamide, procarbazine and prednisone [bilirubin 0.8 mg/dL], allowing for more aggressive therapy).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Procarbazine-induced hepatotoxicity: case report and review of the literature.[Pharmacotherapy. 2010]Review Procarbazine-induced hepatotoxicity: case report and review of the literature.Fesler MJ, Becker-Koepke S, Di Bisceglie AM, Petruska PJ. Pharmacotherapy. 2010 May; 30(5):540.
- Procarbazine for non-Hodgkin's lymphoma.[Leuk Lymphoma. 2006]Procarbazine for non-Hodgkin's lymphoma.Chaar BT, Salem P, Petruska PJ. Leuk Lymphoma. 2006 Apr; 47(4):637-40.
- Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma.[Ann Hematol. 1994]Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma.Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H. Ann Hematol. 1994 Mar; 68(3):105-10.
- NATULAN IN MANAGEMENT OF LATE HODGKIN'S DISEASE, OTHER LYMPHORETICULAR NEOPLASMS, AND MALIGNANT MELANOMA.[Br Med J. 1965]NATULAN IN MANAGEMENT OF LATE HODGKIN'S DISEASE, OTHER LYMPHORETICULAR NEOPLASMS, AND MALIGNANT MELANOMA.TODD ID. Br Med J. 1965 Mar 6; 1(5435):628-31.
- Review Lomustine.[LiverTox: Clinical and Researc...]Review Lomustine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Procarbazine - LiverToxProcarbazine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...