NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Probenecid is a uricosuric agent used for the treatment of gout usually in combination with other agents. Probenecid has been associated with minor serum aminotransferase elevations and very rarely with hypersensitivity reactions which, even more rarely, can be accompanied by acute liver injury.
Background
Probenecid (proe ben' e sid) is a sulfonamide derivative that acts as an inhibitor of inorganic acid transport in the distal renal tubule, causing blockage of reabsorption of uric acid. Therapy leads to lowering of serum uric acid levels within a few weeks, and chronic therapy has been shown to decrease uric acid levels and to decrease acute gouty attacks. Probenecid also inhibits the excretion of penicillin and other drugs and has been used as an adjunct to penicillin therapy to increase plasma drug levels. Probenecid was approved for use in the United States in 1951 and is still used for therapy and prevention of gout and hyperuricemia. Probenecid is available in tablets of 500 mg in several generic forms and under brand names such as Benemid and Benuryl, as well as in fixed combinations with colchicine. The recommended initial dose for gout is 250 to 500 mg twice a day, which can be increased to 2 g daily based upon target levels of uric acid. Probenecid is typically used in combination with other agents for gout, such as colchicine. Common side effects include headache, gastrointestinal upset, hypersensitivity reactions and transient worsening of gout.
Probenecid is also used in combination with penicillin and penicillin derivatives (such as ampicillin and nafcillin) to prolong their plasma half-life and increase serum concentrations. The typical dose for this effect is 1 g daily. Importantly, however, probenecid has similar effects on many other medications, inhibiting the tubular secretion and increasing serum concentrations of drugs such as acetaminophen, naproxen, indomethacin, ketoprofen, lorazepam and rifampin. Thus, patients taking probenecid should be cautioned about use of other medications.
Hepatotoxicity
There are no reports on the frequency of liver test abnormalities during probenecid therapy, but they are probably rare as the drug is largely secreted unchanged in the urine. A single case report of a severe hypersensitivity reaction from probenecid and rechallenge with a rapid and severe recurrence of jaundice was reported over 50 years ago. As is typical for hypersensitivity reactions, the onset was within days of starting probenecid and was accompanied by fever and rash.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of probenecid hepatotoxicity is probably hypersensitivity. Most cases of hypersensitivity to probenecid are marked by skin rash alone, without liver damage.
Outcome and Management
Chronic hepatitis and vanishing bile duct syndrome have not been reported from probenecid therapy. Hypersensitivity reactions (largely rash and urticaria) to probenecid can occur and rechallenge should be avoided as the single case of severe liver injury reported was in a patient who was retreated with probenecid after a prolonged hypersensitivity reaction to a previous exposure.
Drug Class: Antigout Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Probenecid – Generic, Benemid®
DRUG CLASS
Antigout Agents/Gout Suppressants
Product labeling at DailyMed, National Library of Medicine, NIH
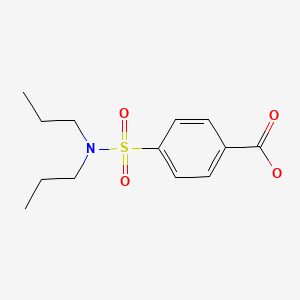
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Probenecid | 57-66-9 | C13-H19-N-O4-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 July 2020
- Zimmerman HJ. Drugs used to treat gout. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 543-4.(Textbook of hepatotoxicity published in 1999; mentions that probenecid has effects on indocyanine green [ICG] and sulfobromophthalein [BSP] excretion, but rarely causes liver injury; despite years of use only a single case report of severe hypersensitivity with jaundice has been published).
- Grosser T, Smyth EM, FitzGerald GA. Pharmacotherapy of inflammation, fever, pain, and gout. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 685-710.(Textbook of pharmacology and therapeutics).
- Reynolds ES, Schlant RC, Gonick HC, Dammin GJ. Fatal massive necrosis of the liver as a manifestation of hypersensitivity to probenecid. N Engl J Med. 1957;256:592–6. [PubMed: 13451901](57 year old man developed fever, rash, eosinophilia and jaundice 2 days after starting probenecid, with slow recovery upon withdrawal and recurrence of fever and jaundice upon restarting, whereupon drug was continued for 10 days when he was admitted with acute liver failure; autopsy showed massive necrosis and portal inflammation without fibrosis).
- Hillecke NA. Acute anaphylactoid reaction to probenecid. JAMA. 1965;193:740. [PubMed: 14328478](61 year old man developed anaphylaxis after single dose of colchicine-probenecid with leukemoid reaction, hypotension and fever, recovering within a week; rechallenge with probenecid without colchicine resulted in recurrence of anaphylaxis [bilirubin not given, AST 80 U/L]).
- Vogin EE, Scott W, Boyd J, Bear WT, Mattis PA. Effect of probenecid on indocyanine green clearance. J Pharmacol Exp Ther. 1966;152:509–15. [PubMed: 5922315](In beagles, probenecid caused a reversible and dose related inhibition of indocyanine green excretion and increase in bile flow, probably due to its known effects on membrane transport).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to probenecid).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to probenecid).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Uricosuric medications for chronic gout.[Cochrane Database Syst Rev. 2014]Uricosuric medications for chronic gout.Kydd AS, Seth R, Buchbinder R, Edwards CJ, Bombardier C. Cochrane Database Syst Rev. 2014 Nov 14; (11):CD010457. Epub 2014 Nov 14.
- Comparative efficacy and safety of uricosuric agents in the treatment of gout or hyperuricemia: a systematic review and network meta-analysis.[Clin Rheumatol. 2023]Comparative efficacy and safety of uricosuric agents in the treatment of gout or hyperuricemia: a systematic review and network meta-analysis.Li YJ, Chen LR, Yang ZL, Wang P, Jiang FF, Guo Y, Qian K, Yang M, Yin SJ, He GH. Clin Rheumatol. 2023 Jan; 42(1):215-224. Epub 2022 Aug 29.
- Review A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients?[Drug Saf. 2008]Review A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients?Lee MH, Graham GG, Williams KM, Day RO. Drug Saf. 2008; 31(8):643-65.
- Review The history and future of probenecid.[Cardiovasc Toxicol. 2012]Review The history and future of probenecid.Robbins N, Koch SE, Tranter M, Rubinstein J. Cardiovasc Toxicol. 2012 Mar; 12(1):1-9.
- The search for uricosuric agents suited to the management of tophaceous gout.[N Y State J Med. 1963]The search for uricosuric agents suited to the management of tophaceous gout.GUTMAN AB. N Y State J Med. 1963 Feb 15; 63:576-85.
- Probenecid - LiverToxProbenecid - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...