NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Piperacillin is parenterally administered, extended-spectrum ureidopenicillin which, when combined with the beta-lactamase inhibitor tazobactam, is used to treat moderate-to-severe infectious due to susceptible organisms including lactamase producing penicillin-resistant bacteria. Piperacillin-tazobactam has been linked with idiosyncratic liver injury, including cases of DRESS and cholestatic liver injury that can be prolonged and even fatal.
Background
Piperacillin-tazobactam is the combination of a fourth generation, extended-spectrum penicillin and a beta-lactamase inhibitor that is used for moderate-to-severe infections caused by susceptible agents, such as (but not limited to) Escherichia coli, many Bacteroides and Klebsiella species, Staphylococcus aureus, and Hemophilus influenzae. The combination of piperacillin with tazobactam provides broad activity against beta-lactamase producing penicillin-resistant bacterial species. This combination was approved for use in the United States in 1985 and is generally reserved for severe infections requiring parenteral therapy. Piperacillin-tazobactam is available in parenteral form for intravenous use in generic forms and under the trade name Zosyn. Recommended doses are 3 to 4.5 grams of piperacillin with 0.375 to 0.5 grams of tazobactam every 6 to 8 hours for 7 to 14 days. Common side effects include headache, dizziness, nausea, diarrhea, constipation, skin rash and hypersensitivity reactions. Rare but potentially severe adverse events include anaphylactic and hypersensitivity reactions, Stevens Johnson syndrome and toxic epidermal necrolysis.
Hepatotoxicity
In large clinical trials, ALT elevations were reported in 6% to 15% and bilirubin elevations in 3% to 5% of patients receiving piperacillin-tazobactam, with considerably lower rates in patients receiving comparator antibiotics (such as imipenim-cilastatin) and lower rates reported with piperacillin alone. These abnormalities were reported to resolve quickly with stopping therapy. Rare instances of idiosyncratic liver injury have been reported in persons receiving the piperacillin without tazobactam and a similar pattern of injury has been reported with the combination. The injury is typically cholestatic or mixed and arises 1 to 3 weeks after starting and often days or weeks after stopping the antibiotic. The liver injury is usually self-limited but severe cases of cholestatic hepatitis can lead to prolonged jaundice or persistence of minor alkaline phosphatase elevations for 6 to 12 months after clinical resolution. Immunoallergic features may be present but are generally mild.
In addition, the combination of piperacillin and tazobactam has been implicated in more than a dozen cases of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, usually arising after 2 to 3 weeks of long term therapy initially with rash and fever followed by liver or renal dysfunction or both. The liver injury is usually mixed or cholestatic but hepatocellular instances have also been reported. In many cases the liver injury is mild and overshadowed by the systemic symptoms and rash. Piperacillin-tazobactam has also been linked to instances of Stevens Johnson syndrome, although in most cases, other drugs known to be associated with the syndrome were being taken. These forms of hypersensitivity with liver involvement have not been reported with piperacillin alone. Because piperacillin-tazobactam is used for serious infections, patients are often taking multiple medications including other antibiotics such as vancomycin, azithromycin or ciprofloxacin, making the causality assessment challenging.
Likelihood score: B (likely rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury associated with piperacillin use is probably hypersensitivity or allergy. Cases of reoccurrence on reexposure have been reported. The role of tazobactam in the injury is uncertain, but it appears to be important in cases of DRESS syndrome.
Outcome and Management
In the few cases of delayed cholestatic hepatitis that have been described with piperacillin with or without tazobactam, patients have recovered within a few weeks of stopping. Cases with DRESS syndrome tend to be more prolonged and are usually treated with corticosteroids for the manifestations of fever and rash. Whether corticosteroids also improve the liver and renal involvement is not clear, but they are generally continued for at least 6 to 8 weeks. Rechallenge is usually followed by recurrence of injury which can be more severe. Patients with piperacillin induced hepatitis should avoid reexposure and should take other penicillins and cephalosporins with caution.
Drug Class: Antiinfective Agents, Penicillins (Fourth Generation)
Other Drugs in the Class: Piperacillin, Ticarcillin, Ticarcillin-Clavulanate
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Piperacillin-Tazobactam – Generic, Zosyn®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Piperacillin-Tazobactam | 157044-21-8 | C23-H27-N5-O7-S. C10-H12-N4-O5-S.Na |
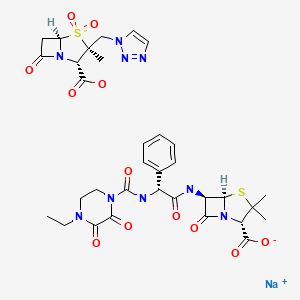
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; piperacillin and ticarcillin are listed as associated with elevations in aminotransferase levels, but without reports of clinically apparent liver injury except with ticarcillin-clavulanate).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that the extended-spectrum penicillins have rarely been associated with clinically apparent liver injury).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Winston DJ, Murphy W, Young LS, Hewitt WL. Piperacillin therapy for serious bacterial infections. Am J Med. 1980;69:255–61. [PubMed: 6447455](Piperacillin was given to 59 patients with severe infections; eosinophilia occurred in 6 and AST elevations in 2, none requiring discontinuation and all resolving with stopping).
- Russo J Jr, Russo ME. Comparative review of two new wide-spectrum penicillins: mezlocillin and piperacillin. Clin Pharm. 1982;1:207–16. [PubMed: 6224627](Review of two extended-spectrum penicillins, piperacillin is more active than mezlocillin against Pseudomonas, side effect profiles are similar including ALT elevations).
- Gooding PG, Clark BJ, Sathe SS. Piperacillin: a review of clinical experience. J Antimicrob Chemother. 1982;9 Suppl B:93–9. [PubMed: 6460738](Review of registration trials including 493 courses of piperacillin, averaging 11 days: 90% cure, 4% hypersensitivity, 6% eosinophilia, 3% transient ALT elevations, 3% bilirubin elevations; one patient developed cholestatic hepatitis that recurred more severely with rechallenge).
- Marier RL, Sanders CV, Faro S, et al. Piperacillin v Carbenicillin in the therapy for serious infections. Arch Intern Med. 1982;142:2000–5. [PubMed: 6215008](Study of 165 hospitalized patients receiving either piperacillin or carbenicillin for serious infections; 95% vs 88% were cured, but liver test abnormalities occurred in 2% with piperacillin vs 22% with carbenicillin; 4% of patients were withdrawn, but none for jaundice).
- Sharifi R, Lee M, Ojeda L. Comparative efficacy of piperacillin versus carbenicillin for complicated urinary tract infections. Urol Int. 1984;39:345–51. [PubMed: 6395464](Randomized clinical trial in complicated urinary tract infections for average of 7 days; mild ALT increases in 4% of 24 piperacillin recipients, but 35% [ALT 64-628 U/L, normal <27] of 17 carbenicillin recipients; no patient required early stopping).
- Lang R, Lishner M, Ravid M. Adverse reactions to prolonged treatment with high doses of carbenicillin and ureidopenicillins. Rev Infect Dis. 1991;13:68–72. [PubMed: 2017635](Retrospective review of 63 patients who received carbenicillin or ureidopenicillins reported high rate of ALT elevations with Mezlocillin [20%] and one cause of jaundice, but none with carbenicillin or piperacillin).
- Hargreaves JE, Herchline TE. Severe cholestatic jaundice caused by mezlocillin. Clin Infect Dis. 1992;15:179–80. [PubMed: 1617065](Case with onset of jaundice 15 days after starting mezlocillin, vancomycin and gentamicin [bilirubin 10 mg/dL, ALT 100 U/L, Alk P 960 U/L]; values worsened when switched to ampicillin, resolved once antibiotics were stopped).
- Ryan J, Dudley FJ. Cholestasis with ticarcillin-potassium clavulanate (Timentin). Med J Aust. 1992;156:291. [PubMed: 1738336](75 year old man developed jaundice 31 days after stopping a 10 day course of ticarcillin-clavulanate [bilirubin 8.1 mg/dL, ALT 448 U/L, Alk P 1330 U/L]; died of progressive lymphoma soon thereafter; resembled liver injury associated with amoxicillin-clavulanate).
- Kuye O, Teal J, DeVries VG, Morrow CA, Tally FP. Safety profile of piperacillin/tazobactam in phase I and III clinical studies. J Antimicrob Chemother. 1993;31 Suppl A:113–24. [PubMed: 8383652](Among 845 subjects in phase III studies, ALT elevations occurred in 12.2% and bilirubin elevations in 4.6% with iv piperacillin-tazobactam; higher than reported with comparators imipenem and aminoglycosides).
- Wise R. The efficacy and safety of piperacillin/tazobactam in the therapy of bacteraemia. J Antimicrob Chemother. 1993;31 Suppl A:97–104. [PubMed: 8383659](Analysis of 142 patients treated in phase II-III studies found ALT elevations in 12% and bilirubin elevations in 9% of recipients of piperacillin-tazobactam, but no report of clinically apparent liver injury or withdrawal for liver abnormalities).
- Arguedas A, Sifuentes-Osornio J, Loaiza C, Herrera M, Corrales JC, Mohs E. An open, multicenter clinical trial of piperacillin/tazobactam in the treatment of pediatric patients with intra-abdominal infections. J Chemother. 1996;8:130–6. [PubMed: 8708744](Analysis of 60 children with peritonitis receiving iv piperacillin-tazobactam for 1-15 days; 8% had ALT and 8% Alk P elevations, but all mild and resolving rapidly with stopping).
- Quattropani C, Schneider M, Helbling A, Zimmermann A, Krähenbühl S. Cholangiopathy after short-term administration of piperacillin and imipenem/cilastatin. Liver. 2001;21:213–6. [PubMed: 11422785](20 year old man received one dose of piperacillin and 3 days of imipenem-cilastatin and developed symptomatic liver disease 2 weeks later[bilirubin 0.9 rising to 4.7 mg/dL, ALT 345- 669 U/L, Alk P 201-559 U/L], resolving within 2 months of stopping; unclear whether injury was due to piperacillin or imipenem: Case #1 in piperacillin record).
- Dietze MA, Martin P, Schaaf-Lafontaine N. Rev Med Liege. 2002;57:571–4. [Clinical case of the month. Cholestatic hepatitis after administration of piperacillin] French. [PubMed: 12440344](58 year old woman developed cholestatic hepatitis 12 days after starting 10 day course of piperacillin, but also 14 after 2 days of amoxicillin-clavulanate [peak bilirubin 15.0 mg/dL, ALT 624 U/L, Alk P 1730 U/L], resolving within 2 months).
- Tan JS, Wishnow RM, Talan DA, Duncanson FP, Norden CW. Treatment of hospitalized patients with complicated skin and skin structure infections: double-blind, randomized, multicenter study of piperacillin-tazobactam versus ticarcillin-clavulanate. The Piperacillin/Tazobactam Skin and Skin Structure Study Group. Antimicrob Agents Chemother. 1993;37:1580–6. [PMC free article: PMC188023] [PubMed: 8215266](Among 251 patients treated with either of 2 fourth generation penicillins, overall rates of response and side effects were similar; no mention of ALT levels of hepatic adverse events).
- Schoonover LL, Occhipinti DJ, Rodvold KA, Danziger LH. Piperacillin/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. Ann Pharmacother. 1995;29:501–14. [PubMed: 7655135](Review of pharmacology, efficacy and safety of piperacillin-tazobactam; the most common side effects are diarrhea [8%], nausea, headache, pruritus and rash [1%]; laboratory abnormalities occur in <1% of patients, but can include "transient increases in liver function test results"; no other mention of hepatotoxicity).
- Dietze MA, Martin P, Schaaf-Lafontaine N. Rev Med Liege. 2002;57:571–4. [Clinical case of the month. Cholestatic hepatitis after administration of piperacillin] French. [PubMed: 12440344](58 year old woman developed cholestatic hepatitis 12 days after starting 10 day course of piperacillin, but also 14 days after finishing a 2 day course of amoxicillin-clavulanate [peak bilirubin 15.0 mg/dL, ALT 624 U/L, Alk P 1730 U/L], resolving within 2 months).
- Yellin AE, Johnson J, Higareda I, Congeni BL, Arrieta AC, Fernsler D, West J, et al. Ertapenem or ticarcillin/clavulanate for the treatment of intra-abdominal infections or acute pelvic infections in pediatric patients. Am J Surg. 2007;194:367–74. [PubMed: 17693284](Among 105 children in a controlled trial, ALT elevations occurred in 3% on ertapenem vs 4% on ticarcillin-clavulanate; no details given).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, two cases were attributed to amoxicillin, but none were attributed to ticarcillin or piperacillin).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to an extended spectrum penicillin).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, but ticarcillin and piperacillin were not listed in the top 41 causes).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to an extended spectrum penicillin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin-clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric; none were attributed to a 4th generation penicillin).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 persons with suspected drug induced liver injury seen over at 12 month period at a referral hospital in Tasmania, 11 were attributed to antibiotics including 4 to flucloxacillin, 2 amoxicillin with clavulanate, 2 amoxicillin, and 1 each to rifampin, moxifloxacin and ciprofloxacin; none to 4th generation penicillins).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury from various classes of agents, mentions that amoxicillin-clavulanate is the leading cause of drug induced liver injury, marked by a latency of several days to weeks, often after stopping the antibiotic, the injury varying from cholestatic to hepatocellular and the mortality rate being as high as 7%; no discussion of the 4th generation penicillins).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin-clavulanate, but none to 4th generation penicillins such as piperacillin or ticarcillin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to a 1st, three to a 2nd [all due to oxacillin], 97 to a 3rd [91 to amoxicillin-clavulanate, and 6 to amoxicillin alone] and five to a 4th generation penicillin [all 5 to piperacillin-tazobactam]).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses).
- Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. [PMC free article: PMC4783956] [PubMed: 26861310](Review of the most common causes of drug induced liver injury based upon categorization from LiverTox, amoxicillin-clavulanate but not other forms of penicillin were in the top category of causes of liver injury [likelihood scores of A, with more than 100 cases published in the literature]).
- Faulkner L, Gibson A, Sullivan A, Tailor A, Usui T, Alfirevic A, Pirmohamed M, et al. Detection of primary T cell responses to drugs and chemicals in HLA-typed volunteers: implications for the prediction of drug immunogenicity. Toxicol Sci. 2016;154:416–29. [PubMed: 27637899](Demonstration of T cell priming to drugs linked with specific class I HLA-alleles).
- Nicoletti P, Aithal GP, Björnsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Cabañas R, Calderon O, Ramirez E, Fiandor A, Prior N, Caballero T, Herránz P, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24:425–30. [PubMed: 25668894](Eight patients with DRESS syndrome attributed to piperacillin-tazobactam were identified at a single Spanish referral center in Madrid between 2006 and 2012; 3 women, 5 men, ages 39 to 83 years, all with rash and eosinophilia, 6 with liver involvement [mixed or cholestatic injury] but none with jaundice, 3 with renal involvement, all survived).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients who underwent outpatient parenteral antibiotic therapy for at least 2 weeks, 210 [25%] developed eosinophilia including 58 of 207 [28%] who received “penicillins” of whom 3 developed signs of “possible” DRESS syndrome; specific penicillins accounting for the cases were not provided).
- Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. [PubMed: 26614821](Review of mechanisms of idiosyncratic drug induced liver injury focusing upon chemically reactive drug metabolites and genetic associations, particularly those with HLA alleles that implicate the adaptive immune response).
- Kraleti S, Khatri N, Jarrett D. Piperacillin-tazobactam induced interstitial nephritis, hepatitis and serum sickness-Like Illness. J Ark Med Soc. 2016;112:278–80. [PubMed: 27434982](30 year old woman developed fever and rash during intravenous therapy with vancomycin and piperacillin-tazobactam with accompanying liver and kidney disfunction and leukocytosis [bilirubin 2.3 mg/dL, ALT 180 U/L, Alk P 708 U/L, INR 2.5, creatinine 5.4], responding rapidly to stopping therapy and treatment with corticosteroids).
- Patel J, Walayat S, Kalva N, Palmer-Hill S, Dhillon S. Bile cast nephropathy: a case report and review of the literature. World J Gastroenterol. 2016;22:6328–34. [PMC free article: PMC4945990] [PubMed: 27468221](54 year old man developed jaundice 2 weeks after starting intravenous piperacillin-tazobactam for osteomyelitis with progressive jaundice and renal dysfunction [bilirubin 19.3 rising to 29.0 mg/dL, ALT 129 U/L, Alk P 851 U/L, INR 1.4, creatinine 2.1 rising to 5.5 mg/dL], treated with dialysis and ursodiol but after six months of progressive disease underwent successful, combined renal and liver transplant).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Björnsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin-clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Piperacillin/tazobactam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential.[Drugs. 1994]Review Piperacillin/tazobactam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential.Bryson HM, Brogden RN. Drugs. 1994 Mar; 47(3):506-35.
- Potency and antimicrobial spectrum update for piperacillin/tazobactam (2000): emphasis on its activity against resistant organism populations and generally untested species causing community-acquired respiratory tract infections.[Diagn Microbiol Infect Dis. 2002]Potency and antimicrobial spectrum update for piperacillin/tazobactam (2000): emphasis on its activity against resistant organism populations and generally untested species causing community-acquired respiratory tract infections.Johnson DM, Biedenbach DJ, Jones RN. Diagn Microbiol Infect Dis. 2002 May; 43(1):49-60.
- Comparison of the Treatment Outcome of Piperacillin-Tazobactam versus Carbapenems for Patients with Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in Areas with Low Frequency of Coproduction of OXA-1: a Preliminary Analysis.[Microbiol Spectr. 2022]Comparison of the Treatment Outcome of Piperacillin-Tazobactam versus Carbapenems for Patients with Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in Areas with Low Frequency of Coproduction of OXA-1: a Preliminary Analysis.Hoashi K, Hayama B, Suzuki M, Sakurai A, Takehana K, Enokida T, Takeda K, Ohkushi D, Doi Y, Harada S. Microbiol Spectr. 2022 Aug 31; 10(4):e0220622. Epub 2022 Aug 2.
- Antimicrobial activities of piperacillin alone and in combination with tazobactam against beta-lactamase-producing bacteria.[J Formos Med Assoc. 1991]Antimicrobial activities of piperacillin alone and in combination with tazobactam against beta-lactamase-producing bacteria.Chang SC, Hsu LY, Luh KT, Hsieh WC. J Formos Med Assoc. 1991 Oct; 90(10):947-52.
- Review Piperacillin/tazobactam in the treatment of polymicrobial infections.[Intensive Care Med. 1994]Review Piperacillin/tazobactam in the treatment of polymicrobial infections.Gorbach SL. Intensive Care Med. 1994 Jul; 20 Suppl 3:S27-34.
- Piperacillin-Tazobactam - LiverToxPiperacillin-Tazobactam - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...