NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The fourth generation penicillins are semisynthetic modifications of natural penicillin that have the advantage of an extended spectrum of activity particularly against gram negative bacteria including Pseudomonas, Enterobacter, Proteus and Klebsiella species. The first generation penicillins are bactericidal antibiotics naturally derived from the mold, Penicillium chrysogenum. Their basic structure includes a thiazolidine ring connected to a beta-lactam ring with a variable side chain. Penicillins bind to bacterial proteins and inhibit synthesis of the bacterial cell wall, causing cell lysis particularly in rapidly growing organisms. Bacterial resistance to penicillin is usually mediated by beta-lactamase, an enzyme which destroys the beta-lactam ring of penicillin, rendering it inactive.
The fourth generation penicillins (sometimes referred to as penicillins with extended spectrum of action) like natural penicillin are susceptible to beta-lactamase. The extended spectrum penicillins are used for therapy of moderate to severe urinary, respiratory, gastrointestinal tract, skin, bone and joint infections. They have activity against Escherichia coli, Hemophilus influenzae, Listeria monocytogenesis, Neisseria gonorrhoeae, Proteus mirabilis, Salmonella, Shigella, Staphylococcus aureus (non-penicillinase producing), Staphylococcus epidermidis, and Streptococcus pneumoniae.
Only one fourth generation penicillin is currently available in the United States: piperacillin (pi" per a sil' in). Several others were used in the United States or Europe, but were abandoned or have been withdrawn (ticarcillin, carbenicillin, mezlocillin and azlocillin). Piperacillin is also available in combination with the beta-lactamase inhibitor tazobactam (taz" oh bak' tam), which provides coverage against penicillinase-resistant bacteria. Piperacillin is a rare cause of acute liver injury and appears to share a common pattern of associated liver injury with other penicillins. Carbenicillin (kar" ben i sil' in), mezlocillin (mez" loe sil' in), and ticarcillin (tye” car sil’ in) fourth generation penicillins that have been withdrawn from use, have also been linked to penicillin-like hepatotoxicity. The combination of ticarcillin with clavulanic acid has been associated with injury that resembles the cholestatic hepatitis that follows therapy with amoxicillin-clavulanate.
Drug Class: Antiinfective Agents
The following extended-spectrum penicillins are discussed separately with individual clinical cases and references. The following are links to each drug record:
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Piperacillin | 59703-84-3 | H26-N5-Na-O7-S |
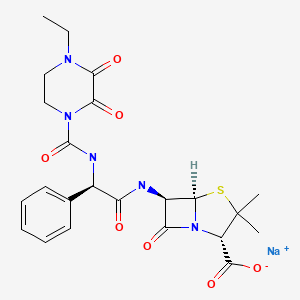
|
| Piperacillin and Tazobactam | 157044-21-8 | C23-H27-N5-O7-S. C10-H12-N4-O5-S.Na |
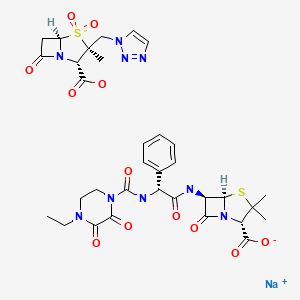
|
| Ticarcillin | 34787-01-4 | C15-H16-N2-O6-S2 |

|
| Ticarcillin and Clavulanic Acid | 86482-18-0 | C15-H16-N2-O6-S2. C8-H9-N-O5 |
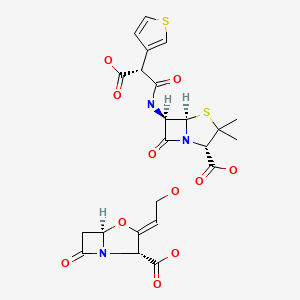
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; piperacillin and ticarcillin are listed as associated with elevations in aminotransferase levels, but without reports of clinically apparent liver injury except with ticarcillin-clavulanate).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that there have been few case reports of liver injury due to the extended penicillins).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Pines A, Khaja G, Raafat H, Sreedharan KS. Preliminary clinical experience with ticarcillin (BRL 2288) in 101 patients treated for severe respiratory infections. Chemotherapy. 1974;20:39–44. [PubMed: 4210892](Early experience in 101 patients with severe infections given intramuscular ticarcillin; local pain was common and there were minimal and transient elevations in ALT in 4 and Alk P is 5 patients).
- Nelson JD, Kusmiesz H, Shelton S, Woodman E. Clinical pharmacology and efficacy of ticarcillin in infants and children. Pediatrics. 1978;61:858–63. [PubMed: 673548](Among 98 children treated with ticarcillin intravenously or intramuscularly, 3 had AST elevations [50-81 U/L] without symptoms or jaundice, and all values decreased during continued treatment).
- Brogden RN, Heel RC, Speight TM, Avery GS. Ticarcillin: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980;20:325–52. [PubMed: 7002527](Ticarcillin is a semisynthetic penicillin for parenteral administration similar to carbenicillin; adverse events include skin rash, hypokalemia, eosinophilia and platelet dysfunction; ALT elevations and hepatic injury are not mentioned).
- Russo J Jr, Russo ME. Comparative review of two new wide-spectrum penicillins: mezlocillin and piperacillin. Clin Pharm. 1982;1:207–16. [PubMed: 6224627](Review of two extended spectrum penicillins, piperacillin is more active than mezlocillin against Pseudomonas, side effect profiles are similar including ALT elevations that occur with both).
- González MA. Comparison of the efficacy and safety of mezlocillin and ticarcillin in the treatment of patients with serious systemic infections. J Antimicrob Chemother. 1982;9 Suppl A:229–30. [PubMed: 6210671](Randomized clinical trial of mezlocillin vs ticarcillin in 34 patients with severe infections; no mention of ALT elevations).
- Parry MF, Neu HC. The safety and tolerance of mezlocillin. J Antimicrob Chemother. 1982;9 Suppl A:273–80. [PubMed: 6210679](Analysis of 1148 patients given intravenous mezlocillin for 1 to 52 days; 3.7% had hypersensitivity reactions, 0.9% elevations in ALT, AST or Alk P, but all were reversible and anicteric. In a direct comparison, AST elevations occurred in 1.5% of mezlocillin vs 7.4% of ticarcillin recipients).
- Ramírez-Ronda CH, Gutiérrez J, Bermúdez RH. Comparative effectiveness, safety and tolerance of mezlocillin and ticarcillin: a prospective randomized trial. J Antimicrob Chemother. 1982;9 Suppl A:125–9. [PubMed: 6210660](Randomized clinical trial comparing mezlocillin [n=21] and ticarcillin [n=20]; no ALT elevations occurred).
- Graft DF, Chesney PJ. Use of ticarcillin following carbenicillin-associated hepatotoxicity. J Pediatr. 1982;100:497–9. [PubMed: 7062188](3 children who developed elevations in serum ALT levels during intravenous therapy with oxacillin or carbenicillin were then treated with ticarcillin without recurrence of liver injury).
- Reed WP, Palmer DL. Comparison of azlocillin and ticarcillin in the treatment of urinary tract infection. J Antimicrob Chemother. 1983;11 Suppl B:189–93. [PubMed: 6619028](Randomized clinical trial of azlocillin vs ticarcillin in 35 patients with urinary tract infections, both were highly effective; no mention of ALT elevations or hepatic injury).
- Jackson D, Cockburn A, Cooper DL, Langley PF, Tasker TC, White DJ. Clinical pharmacology and safety evaluation of Timentin. Am J Med. 1985;79(5B):44–55. [PubMed: 4073095](Toxicological studies of ticarcillin and clavulanate in animals and in pharmacologic studies in humans found no evidence of significant liver injury).
- Tasker TC, Cockburn A, Jackson D, Mellows G, White D. Safety of ticarcillin disodium/potassium clavulanate. J Antimicrob Chemother 1986; 17 Suppl C: 225-32. [PubMed: 3722044](Preclinical evaluation of ticarcillin found no evidence of significant hepatic injury; early clinical studies found low rate of elevated serum enzymes during therapy, but these may have been due to the underlying illness being treated).
- Tan JS, Wishnow RM, Talan DA, Duncanson FP, Norden CW. Treatment of hospitalized patients with complicated skin and skin structure infections: double-blind, randomized, multicenter study of piperacillin-tazobactam versus ticarcillin-clavulanate. The Piperacillin/Tazobactam Skin and Skin Structure Study Group. Antimicrob Agents Chemother. 1993;37:1580–6. [PMC free article: PMC188023] [PubMed: 8215266](Among 251 patients treated with either of 2 fourth generation penicillins, overall rates of response and side effects were similar; no mention of ALT levels of hepatic adverse events).
- Schoonover LL, Occhipinti DJ, Rodvold KA, Danziger LH. Piperacillin/tazobactam: a new beta-lactam/beta-lactamase inhibitor combination. Ann Pharmacother. 1995;29:501–14. [PubMed: 7655135](Review of pharmacology, efficacy and safety of piperacillin-tazobactam; the most common side effects are diarrhea [8%], nausea, headache, pruritus and rash [1%]; laboratory abnormalities occur in <1% of patients, but can include "transient increases in liver function test results"; no other mention of hepatotoxicity).
- Yellin AE, Johnson J, Higareda I, Congeni BL, Arrieta AC, Fernsler D, West J, et al. Ertapenem or ticarcillin/clavulanate for the treatment of intra-abdominal infections or acute pelvic infections in pediatric patients. Am J Surg. 2007;194:367–74. [PubMed: 17693284](Among 105 children in a controlled trial, ALT elevations occurred in 3% on ertapenem vs 4% on ticarcillin-clavulanate; no details given).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to an extended spectrum penicillin).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, but ticarcillin and piperacillin were not listed in the top 41 causes).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to an extended spectrum penicillin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin-clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric; none were attributed to a 4th generation penicillin).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 persons with suspected drug induced liver injury seen over at 12 month period at a referral hospital in Tasmania, 11 were attributed to antibiotics including 4 to flucloxacillin, 2 amoxicillin with clavulanate, 2 amoxicillin, and 1 each to rifampin, moxifloxacin and ciprofloxacin; none to 4th generation penicillins).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury from various classes of agents, mentions that amoxicillin-clavulanate is the leading cause of drug induced liver injury, marked by a latency of several days to weeks, often after stopping the antibiotic, the injury varying from cholestatic to hepatocellular and the mortality rate being as high as 7%; no discussion of the 4th generation penicillins).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin-clavulanate, but none to 4th generation penicillins such as piperacillin or ticarcillin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to 1st, three to 2nd [all due to oxacillin], 97 to 3rd [91 to amoxicillin-clavulanate, and 6 to amoxicillin alone] and five to 4th generation penicillins [all 5 to piperacillin-tazobactam]).
- Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Cabañas R, Calderon O, Ramirez E, Fiandor A, Prior N, Caballero T, Herránz P, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24:425–30. [PubMed: 25668894](Eight patients with DRESS syndrome attributed to piperacillin-tazobactam were identified at a single Spanish referral center in Madrid between 2006 and 2012; 3 women, 5 men, ages 39 to 83 years, all with rash and eosinophilia, 6 with liver involvement [mixed or cholestatic injury] but none with jaundice, 3 with renal involvement, all survived).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients who underwent outpatient parenteral antibiotic therapy for at least 2 weeks, 210 [25%] developed eosinophilia including 58 of 207 [28%] who received “penicillins” of whom 3 developed signs of “possible” DRESS syndrome; specific penicillins accounting for the cases were not provided).
- Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. [PubMed: 26614821](Review of mechanisms of idiosyncratic drug induced liver injury focusing upon chemically reactive drug metabolites and genetic associations, particularly those with HLA alleles that implicate the adaptive immune response).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Penicillins (3rd Generation).[LiverTox: Clinical and Researc...]Review Penicillins (3rd Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review The penicillins.[Mayo Clin Proc. 1991]Review The penicillins.Wright AJ, Wilkowske CJ. Mayo Clin Proc. 1991 Oct; 66(10):1047-63.
- Review The penicillins.[Mayo Clin Proc. 1999]Review The penicillins.Wright AJ. Mayo Clin Proc. 1999 Mar; 74(3):290-307.
- Review Penicillins (1st Generation).[LiverTox: Clinical and Researc...]Review Penicillins (1st Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Comparative in vitro activities of piperacillin-tazobactam and ticarcillin-clavulanate.[Antimicrob Agents Chemother. 1...]Comparative in vitro activities of piperacillin-tazobactam and ticarcillin-clavulanate.Fass RJ, Prior RB. Antimicrob Agents Chemother. 1989 Aug; 33(8):1268-74.
- Penicillins (4th Generation) - LiverToxPenicillins (4th Generation) - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...