NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Piperacillin is an extended spectrum ureidopenicillin and is used to treat moderate-to-severe infections due to susceptible organisms. Piperacillin has been linked with idiosyncratic liver injury, but only rarely and in isolated case reports.
Background
Piperacillin (pi" per a sil' in) is a fourth generation, extended spectrum penicillin which is used for moderate-to-severe infections caused by susceptible agents, such as (but not limited to) Escherichia coli, Hemophilus influenzae, Listeria monocytogenesis, Neisseria gonorrhoeae, Proteus mirabilis, Salmonella, Shigella, Staphylococcus aureus (non-penicillinase producing), Staphylococcus epidermidis, and Streptococcus pneumoniae. Piperacillin was approved for use in the United States in 1981 and is generally reserved for severe infections requiring parenteral therapy. Piperacillin is available in parenteral form (intramuscular and intravenous) generically and under the trade name Pipracil. Piperacillin is often combined with a beta lactamase inhibitor (tazobactam) to prevent bacterial resistance. The recommended regimen is 2 to 4 grams daily in divided doses given every 4 to 6 hours usually for 5 to 7 days. Common side effects include headache, dizziness, nausea, diarrhea, constipation, skin rash and hypersensitivity reactions. Rare but potentially severe adverse events include anaphylactic and hypersensitivity reactions, Stevens Johnson syndrome and toxic epidermal necrolysis.
Hepatotoxicity
Patients on intravenous piperacillin may have transient and mild-to-moderate serum aminotransferase elevations in up to 12% of patients, but these are of little clinical significance and not more common than with comparative parenteral antibiotics. Hepatic injury was more commonly reported with mezlocillin, a related extended spectrum ureidopenicillin which has been withdrawn from use. Rare instances of idiosyncratic liver injury have been reported in persons receiving piperacillin. The liver injury is typically cholestatic arising within 1 to 6 weeks of starting therapy. The injury can be severe, but is generally self-limited once piperacillin is stopped. The features of the hepatotoxicity resemble those of other penicillins. The cholestatic hepatitis caused by piperacillin and other penicillins can be prolonged and lead to persistent cholestasis (vanishing bile duct syndrome) or persistent elevations in serum alkaline phosphatase suggestive of partial bile duct loss. Most cases of liver injury related to piperacillin are linked to the combination of piperacillin with the beta-lactamase inhibitor tazobactam (Zosyn and generics), which is more commonly used than piperacillin alone.
Likelihood score: B (known rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury associated with piperacillin use is probably hypersensitivity or allergy. Instances of cross reactivity with other penicillins and worsening of cholestasis with reintroduction of the agent have been reported.
Outcome and Management
In the few cases that have been described, patients have recovered within 1 to 3 months once the drug is stopped. Patients with piperacillin induced hepatitis should avoid reexposure to other penicillins and should take cephalosporins with caution.
References to the safety and potential hepatotoxicity of piperacillin are provided in the drug record on Piperacillin-Tazobactam.
Drug Class: Antiinfective Agents, Penicillins (Fourth Generation)
Other Drugs in the Class: Piperacillin-Tazobactam, Ticarcillin, Ticarcillin-Clavulanate
CASE REPORT
Case 1. Cholangiopathy with possible causation by piperacillin.(1)
A 20 year old man underwent surgery for abdominal trauma, receiving a single dose of piperacillin and 3 days of imipenem-cilastatin postoperatively and subsequently developed fever, fatigue, and pruritus starting 15 days after surgery. He had moderate elevations in serum aminotransferase and alkaline phosphatase levels and serum bilirubin subsequently rose and peaked at 4.8 mg/dL (Table). He had no history of liver disease or risk factors for viral hepatitis. He had not used alcohol and took no other medications after surgery. He was admitted for evaluation. Ultrasound and CT scan of the abdomen were negative. Tests for viral hepatitis and autoimmune liver disease were negative. A liver biopsy showed intrahepatic cholestasis with portal inflammation. Lymphocyte transformation tests were reported to be positive with both piperacillin and imipenem-cilastatin. He recovered rapidly and all tests had returned to normal when seen two months later.
Key Points
| Medication: | Piperacillin, 1 gram IV bolus |
|---|---|
| Pattern: | Mixed (R=4.9) |
| Severity: | 3+ (jaundiced and hospitalization) |
| Latency: | 15 days to symptoms, 22 days to jaundice |
| Recovery: | ~2 months |
| Other medications: | Imipenem-cilastatin (4 g daily IV for 3 days) and acetaminophen (4 g daily orally for 3 days) in the postoperative period. |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 210 | 90 | Surgery | ||
| Emergency surgery small bowel perforation: one dose of piperacillin given IV | |||||
| 4 days | 3 days | 83 | 39 | Discharged | |
| Developed fatigue followed by pruritus ~day 15 after surgery | |||||
| 21 days | 20 days | 345 | 201 | 0.9 | |
| 22 days | 21 days | 654 | 416 | 2.9 | |
| 25 days | 24 days | 669 | 559 | 4.7 | Liver biopsy |
| 35 days | 34 days | 210 | 531 | 1.2 | |
| 3months | 3 months | 13 | 79 | 0.8 | |
| Normal Values | <37 | <108 | <1.2 | ||
Comment
The young man clearly had drug induced liver disease with a mixed pattern of serum enzyme elevations and mild cholestasis that resolved completely in follow up. The difficulty is knowing which agent or agents was responsible. He received only one dose of piperacillin and less than 3 days of imipenem/cilastatin. Neither of these agents are common causes of drug induced liver disease. One might also question whether a halogenated anesthetic was used that might have caused liver injury and whether acetaminophen may have contributed. Hepatotoxicity from these agents, however, is usually more rapid in onset and hepatocellular in character. The pattern of injury was most compatible with a penicillin induced hepatic reaction. Lymphocyte transformation tests may be helpful, but their reliability in discriminating the cause of hepatic injury remains unproven; in this instance reactivity was found with both agents. This patient should probably be advised not to receive penicillin in the future.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Piperacillin – Generic, Pipracil®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Piperacillin | 59703-84-3 | H26-N5-Na-O7-S |
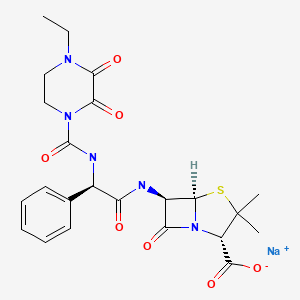
|
CITED REFERENCE
- 1.
- Quattropani C, Schneider M, Helbling A, Zimmermann A, Krähenbühl S. Cholangiopathy after short-term administration of piperacillin and imipenem/cilastatin. Liver. 2001;21:213–6. [PubMed: 11422785]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Piperacillin-Tazobactam.[LiverTox: Clinical and Researc...]Review Piperacillin-Tazobactam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ticarcillin.[LiverTox: Clinical and Researc...]Review Ticarcillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Piperacillin/tazobactam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential.[Drugs. 1994]Review Piperacillin/tazobactam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential.Bryson HM, Brogden RN. Drugs. 1994 Mar; 47(3):506-35.
- In vitro susceptibility of multi-drug resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from clinical specimens at Bugando Medical Centre, Tanzania to Piperacillin-Tazobactam.[Tanzan J Health Res. 2014]In vitro susceptibility of multi-drug resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from clinical specimens at Bugando Medical Centre, Tanzania to Piperacillin-Tazobactam.Petro D, Mushi MF, Moremi N, Iddi S, Mirambo M, Seni J, Mshana SE. Tanzan J Health Res. 2014 Jan; 16(1):54-7.
- Potency and antimicrobial spectrum update for piperacillin/tazobactam (2000): emphasis on its activity against resistant organism populations and generally untested species causing community-acquired respiratory tract infections.[Diagn Microbiol Infect Dis. 2002]Potency and antimicrobial spectrum update for piperacillin/tazobactam (2000): emphasis on its activity against resistant organism populations and generally untested species causing community-acquired respiratory tract infections.Johnson DM, Biedenbach DJ, Jones RN. Diagn Microbiol Infect Dis. 2002 May; 43(1):49-60.
- Piperacillin - LiverToxPiperacillin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...