NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Omeprazole and esomeprazole are proton pump inhibitors (PPIs) and potent inhibitor of gastric acidity which are widely used in the therapy of gastroesophageal reflux and peptic ulcer disease. Omeprazole and esomeprazole therapy are both associated with a low rate of transient and asymptomatic serum aminotransferase elevations and are rare causes of clinically apparent liver injury.
Background
Omeprazole (oh mep' ra zole), like other PPIs, inhibits gastric acid production by binding to and inactivating the H+/K+-ATPase of gastric parietal cells, causing inhibition of the proton pump that transports H+ into the gastric lumen, the common final step in gastric acid production. Omeprazole is a prodrug and is converted to the active form (sulfenic acid) in the acidic secretory canaliculi of parietal cells. Because the inhibition is irreversible, acid secretion is suppressed for 24 to 48 hours, until new proton pump molecules have been synthesized and transported to the cell membrane. Omeprazole was the first PPI approved for use in the United States (1989), initially only for the indication of severe peptic ulcer disease and Zollinger-Ellison syndrome. Subsequently, the indications for its use have broadened to routine peptic ulcer disease, gastroesophageal reflux disease and prevention of stress ulcers.
Esomeprazole (es" oh mep' ra zole) is the S-isomer of omeprazole and exhibits delayed clearance and thus higher potency on a molar basis than omeprazole, which is a racemic mixture of both S- and R-isomers. The pharmacokinetics, mechanism of action and clinical efficacy of esomeprazole is quite similar to that of omeprazole. Esomeprazole was approved for use in the United States in 2001 with similar indications as omeprazole includig peptic ulcer disease, gastroesophageal reflux disease and prevention of stress ulcers.
Omeprazole and esomeprazole are two of the most widely used medications in clinical practice with more than 20 million prescriptions filled yearly in the United States alone. Omeprazole is available in multiple forms including 10, 20 and 40 mg standard and delayed release capsules and powder for oral suspension in generic forms and under the brand name of Prilosec. The typical dose of omeprazole is 20 mg once daily with twice daily doses for more severe cases of gastrointestinal reflux and peptic ulcer disease, and doses of up to 120 mg daily for Zollinger-Ellison syndrome. A 10 to 14 day course of omeprazole in combination with antibiotics is effective and recommended for the eradication of H. Pylori infection.
Esomeprazole is also available in multiple forms and typically used in 20 mg delayed release capsules generically and under the brand name Nexium. On a molar basis, esomeprazole is more potent than omeprazole and the recommended dose in adults is 20 mg once daily, with twice daily doses for more severe cases of gastrointestinal reflux and peptic ulcer disease, and doses of up to 60 mg daily for Zollinger-Ellison syndrome. Combinations of esomeprazole with antibiotics for 10 to 14 days are effective and recommended for eradication of H. pylori infection. Both omeprazole and esomeprazole are very well tolerated and both are now available without prescription in multiple over-the-counter forms. Side effects of omeprazole and esomeprazole are uncommon and usually mild; they include diarrhea, nausea, vomiting, abdominal discomfort, flatulence, skin rash, headaches and dizziness. Severe side effects are rare but can include hypersensitivity reactions. Long-term use of rabeprazole may be associated with bone fractures, acute interstitial nephritis, lupus erythematosus, vitamin B12 deficiency and hypomagnesemia.
Hepatotoxicity
Despite their wide use, omeprazole and esomeprazole have only rarely been associated with hepatic injury. In large scale, long term trials , serum ALT elevations occurred in less than 1% of patients and at rates similar to those that occurred with placebo or comparator drugs. A small number of cases of clinically apparent liver disease due to omeprazole or esomeprazole have been published, the frequency of these cases probably being less than 1:100,000 users. A somewhat characteristic clinical phenotype has been described, with most cases arising during the first 1 to 4 weeks of therapy and being marked by an acute hepatocellular pattern of injury, with rapid recovery upon withdrawal. Rash, fever and eosinophilia were rare, as is autoantibody formation. Liver biopsy typically shows prominent centrolobular necrosis, suggestive of an acute, toxic hepatic injury (acute hepatic necrosis); however, recurrence upon rechallenge has been documented in several cases. In some instances, other organ involvement is prominent including rhabdomyolysis, lactic acidosis, renal insufficiency or Stevens Johnson syndrome. In large case series of drug induced liver injury, omeprazole and esomeprazole have accounted for few instances of symptomatic acute liver injury and rare instances of acute liver failure.
Likelihood score: B (rare but likely cause of clinically apparent liver injury).
Mechanism of Injury
The acute onset and rapid recurrence of hepatic injury with omeprazole and esomeprazole suggests a hypersensitivity reaction, but may merely reflect altered metabolism or acute toxicity of a metabolic byproduct. The usual clinical phenotype is acute hepatic necrosis. Both omeprazole and esomeprazole are extensively metabolized by the hepatic P450 system and have multiple effects on the drug metabolizing system, including inhibition of CYP 2C19 and induction of CYP1A2, effects which may cause significant drug-drug interactions.
Outcome and Management
The mild and asymptomatic elevations in serum aminotransferase that have been observed during omeprazole and esomeprazole therapy are usually transient and may resolve even without dose modification. Clinically apparent liver injury due to these agents, however, generally calls for prompt withdrawal. Severe injury due to omeprazole and esomeprazole is uncommon and most cases resolve promptly upon withdrawal. Cases of acute liver failure due to proton pump inhibitors have been described, but they are exceedingly rare. There is no information about cross sensitivity to liver injury among the various PPIs, but some degree of cross reactivity should be expected between omeprazole and its S-isomer, esomeprazole. Furthermore, the PPIs all share a benzimidazole structure, and caution should be used in attempting to reintroduce another PPI after clinically apparent hepatic injury due to an unrelated PPI.
Drug Class: Antiulcer Agents
Other Drugs in the Subclass, Proton Pump Inhibitors: Dexlansoprazole, Lansoprazole, Pantoprazole, Rabeprazole
CASE REPORT
Case 1. Acute liver failure attributed to omeprazole.
[Modified from: Jochem V, Kirkpatrick R, Greenson J, Brogan M, Sturgis T, Cook-Glenn C. Fulminant hepatic failure related to omeprazole. Am J Gastroenterol 1992; 87: 523-5. PubMed Citation]
A 62 year old man with gastroesophageal reflux was treated with ranitidine (150 mg twice daily) for 10 days without improvement, whereupon he was switched to omeprazole (20 mg daily). Seventeen days later, he presented with a 4 day history of abdominal pain, nausea, vomiting, poor appetite and weakness. He had no previous history of liver disease, alcohol abuse or risk factors for viral hepatitis. He had a long history of hypertension and coronary artery disease and had been taking atenolol (100 mg daily), diltiazem (60 mg twice daily) and aspirin (325 mg daily) chronically. He denied taking acetaminophen. On examination, he was jaundiced and had mild asterixis without rash or peripheral manifestations of cirrhosis. Laboratory testing showed marked elevations in serum aminotransferase levels (ALT 9234 U/L, AST 2952 U/L), bilirubin (8.4 mg/dL) and prothrombin time (>100 seconds), with minimal increase in alkaline phosphatase levels (254 U/L). Serum lactate was elevated at 26.6 mmol/L and ammonia at 38 µmol/L. The peripheral white blood cell count was elevated (18,100 cells/µL) without eosinophilia and creatinine was 2.6 mg/dL. All liver tests had been normal when measured one month previously. Tests for hepatitis A, B and C were negative. He was managed in the intensive care unit but worsened overnight, becoming obtunded and then comatose. He was transferred to a tertiary medical center for possible liver transplantation, but rapidly developed respiratory and renal failure and died five days after his initial presentation. On autopsy, his liver showed massive centrolobular necrosis without fibrosis.
Key Points
| Medication: | Omeprazole (20 mg daily) |
| Pattern: | Hepatocellular (R=100) |
| Severity: | 5+ (acute liver failure and death) |
| Latency: | 14 days to onset of symptoms |
| Recovery: | None |
| Other medications: | Atenolol, diltiazem and aspirin chronically, ranitidine for 10 days immediately before omeprazole was started |
Comment
This patient developed fulminant hepatic failure and died within 3 weeks of starting omeprazole for gastroesophageal reflux. Spontaneous, idiopathic acute liver failure is very rare, but omeprazole use is common. For these reasons, a person taking omeprazole might develop acute liver failure by chance, unrelated to the medication. However, the onset of liver injury within a few weeks of starting omeprazole and the similarity in time to onset and enzyme pattern to other cases of omeprazole hepatotoxicity suggest that the proton pump inhibitor was the cause. Approximately 10% of causes of hepatocellular jaundice due to medications result in fatality, so that rare instances of proton pump inhibitor induced acute liver injury (which is typically hepatocellular) are likely to result in death. Acute liver failure with a short latency period (1 to 4 weeks) has also been reported with esomeprazole and with rabeprazole therapy.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Omeprazole – Generic, Prilosec®
Esomeprazole – Generic, Nexium®
DRUG CLASS
Antiulcer Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Omeprazole | 73590-58-6 | C17-H19-N3-O3-S |
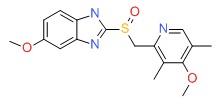 |
| Esomeprazole | 119141-88-7 | C17-H19-N3-O3-S |
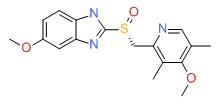 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 April 2019
- Zimmerman HJ. Proton pump inhibitors. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 720-1.(Expert review of hepatotoxicity published in 1999 states that aminotransferase elevations occur in ~1% of PPI treated patients, but only 1 case of acute liver injury has been reported with omeprazole, none with the more recently released lansoprazole and pantoprazole).
- Sharkey KA, McNaughton WK. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 20181, pp. 409-420.(Textbook of pharmacology and therapeutics).
- Gustavsson S, Adami HO, Lööf L, Nyberg A, Nyrén O. Rapid healing of duodenal ulcers with omeprazole: Double-blind dose comparative trial. Lancet 1983; 2: 124-5. [PubMed: 6134979](Controlled trial of two doses of omeprazole in 32 patients with duodenal ulcer reported a high rate of ALT elevations [32%] usually within the first 1-2 weeks, resolving rapidly on withdrawal; peak value 468 U/L).
- Sharma BK, Santana IA, Walt RP, Pounder RE. Omeprazole and liver function tests. Lancet 1983; 2: 346. [PubMed: 6135864](Letter in response to Gustavsson [1983]; among 17 patients with duodenal ulcer treated with omeprazole, none had ALT or Alk P elevations).
- Lööf L, Adami HO, Gustavsson S, Nyberg A, Nyrén O, Lundborg P. Omeprazole: no evidence for frequent hepatic reactions. Lancet 1984; 1: 1347-8. [PubMed: 6145039](Further follow up on the observation of Gustavsson [193], controlled trial of omeprazole in 60 volunteers monitored carefully for ALT elevations, found no association of drug with ALT elevations, being found in 1 of 30 on omeprazole and 2 of 30 on placebo; two patients with previous ALT elevations had no recurrence on rechallenge).
- Lauritsen K, Rune SJ, Bytzer P, Kelbaek H, Jensen KG, Rask-Madsen J, Bendtsen F, et al. Effect of omeprazole and cimetidine on duodenal ulcer. A double-blind comparative trial. N Engl J Med 1985; 312: 958-61. [PubMed: 3883182](Controlled trial of omeprazole vs cimetidine for 4 weeks in 132 patients with duodenal ulcer disease found no major side effects; one patient on omeprazole had rise of Alk P to 756 U/L, resolving with stopping therapy and 4 had minor AST elevations at the end of therapy [36-54 U/L]).
- Sölvell L. Safety aspects of omeprazole. Scand J Gastroenterol Suppl 1986; 118: 129-35. [PubMed: 3460168](Careful analysis of changes in serum ALT levels during omeprazole therapy in prospective studies found little evidence of changes compared to baseline or to placebo treatment; among more than 2500 recipients, only one report of increased ALT and none of hepatitis with jaundice).
- Joelson S, Joelson IB, Lundborg P, Walan A, Wallander MA. Safety experience from long-term treatment with omeprazole. Digestion 1992; 51 (Suppl 1): 93-101. [PubMed: 1397750](Summary of adverse event profile in 2818 patients on long term omeprazole found low rate of side effects, similar in frequency to those on ranitidine or cimetidine; no mention of ALT elevations, hepatitis or jaundice).
- Maton PN, Vinayek R, Frucht H, McArthur KA, Miller LS, Saeed ZA, Gardner JD, et al. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology 1989; 97: 827-36. [PubMed: 2777040](40 patients with Zollinger-Ellison syndrome were treated for 6-51 months with omeprazole in doses of 20-120 mg/day; "No patient developed a side effect that could be attributed to omeprazole").
- Jochem V, Kirkpatrick R, Greenson J, Brogan M, Sturgis T, Cook-Glenn C. Fulminant hepatic failure related to omeprazole. Am J Gastroenterol 1992; 87: 523-5. [PubMed: 1553942](62 year old with reflux which did not improve with ranitidine therapy, developed pain, nausea and fatigue 17 days after starting omeprazole [bilirubin 8.6 mg/dL, ALT 9234 U/L, Alk P 254 U/L, protime >100 seconds], dying 5 days after admission and having massive centrolobular necrosis on autopsy: Case 1).
- Sauvet P, Schouler L. [Omeprazole and liver functions]. Rev Med Interne 1992; 13: 359-63. French. [PubMed: 1344831](Review of the pharmacokinetics and hepatic metabolism of omeprazole and discussion of potential for hepatotoxicity).
- Arnold R. Safety of proton pump inhibitors.an overview. Aliment Pharmacol Ther 1994; 8 Suppl 1: 65-70. [PubMed: 8180297](Side effects are uncommon and no more frequent than with cimetidine and ranitidine; "Omeprazole had no clinically relevant effects of laboratory values including...liver function").
- Klinkenberg-Knol EC, Festen HP, Jansen JB, Lamers CB, Nelis F, Snel P, Luckers A, et al. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med 1994; 121: 161-7. [PubMed: 8017742](91 patients with refractory esophagitis were treated with omeprazole [20-40 mg/day] for 3-5 years; generally well tolerated and no mention of ALT elevations or hepatotoxicity).
- del Rio Fernandez M, Plagaro Cordero ME, de Frutos Arribas JF, Bellido Casado J, Velicia Llanes R. [Hepatic encephalopathy related to omeprazole]. Rev Esp Enferm Dig 1997; 89: 574-5. Spanish. [PubMed: 9303628](49 year old with diabetes, renal transplant, cirrhosis due to hepatitis C developed disorientation and worsening hepatic encephalopathy 6 days after starting omeprazole, recovering with stopping therapy, enemas and lactulose; authors suggest that omeprazole may have precipitated worsening of liver disease and encephalopathy).
- d'Adamo G, Spinelli C, Forte F, Gangeri F. Omeprazole-induced acute interstitial nephritis. Ren Fail 1997; 19: 171-5. [PubMed: 9044464](Case of acute nephritis arising within a few weeks of starting omeprazole; no liver test abnormalities reported).
- Navarro JF, Gallego E, Aviles J. Recurrent severe acute hepatitis and omeprazole. Ann Intern Med 1997; 127: 1135-6. [PubMed: 9412333](30 year old developed abdominal pain 9 days after starting omeprazole [ALT 1294 U/L], resolving in 4 weeks and arising again 1 week after restarting [ALT 762 U/L]; no mention of bilirubin or Alk P levels).
- Christe C, Stoller R, Vogt N. Omeprazole-induced hepatotoxicity? A case report. Pharmacoepidemiol Drug Saf 1998; 7 Suppl 1: S41-4. [PubMed: 15073958](85 year old with congestive heart failure on long term omeprazole developed fatigue and worsening liver tests [bilirubin 2.0 mg/dL, ALT 263 rising to 1236 U/L, LDH 870 to 2100 U/L, Alk P 112 U/L], improving on stopping and with mild recurrence on restarting [ALT 500 U/L], patient dying 10 days later of acute myocardial infarction and heart failure).
- Koury SI, Stone CK, La Charité DD. Omeprazole and the development of acute hepatitis. Eur J Emerg Med 1998; 5: 467-9. [PubMed: 9919455](39 year old found to have abnormal liver tests 4 weeks after starting omeprazole [bilirubin 0.6 mg/dL, ALT 262 U/L, Alk P 103 U/L], resolving within 2 weeks of stopping).
- Koury SI, Stone CK, La Charité DD. Acute hepatitis induced by omeprazole. Am J Emerg Med 1998; 16: 550-1. [PubMed: 9725985](34 year old with chronic hepatitis C and peptic ulcer disease developed abdominal pain and nausea 2 weeks after starting omeprazole [bilirubin 0.3 mg/dL, AST 1059 U/L, Alk P 149 U/L]; rapid improvement on stopping).
- Romero-Gómez M, Otero MA, Suárez-García E, García Díaz E, Fobelo MJ, Castro-Fernández M. Acute hepatitis related to omeprazole. Am J Gastroenterol 1999; 94: 1119-20. [PubMed: 10201510](34 year old developed jaundice 11 days after starting omeprazole [bilirubin 10.4 mg/dL, ALT 1100 U/L, Alk P 461 U/L], resolving in 6 months of stopping).
- Reilly JP. Safety profile of the proton-pump inhibitors. Am J Health Syst Pharm 1999; 56 (23 Suppl 4): S11-7. [PubMed: 10597119](Review of side effects of proton pump inhibitors, including long term tolerance).
- Martin RM, Dunn NR, Freemantle S, Shakir S. The rates of common adverse events reported during treatment with proton pump inhibitors used in general practice in England: cohort studies. Br J Clin Pharmacol 2000; 50: 366-72. [PMC free article: PMC2014999] [PubMed: 11012560](Prescription event monitoring of common side effects of omeprazole, lansoprazole and pantoprazole from the UK found low rates of diarrhea [0.18-0.39/1000 days], abdominal pain [0.17-.21], nausea [0.16-0.22] and headache [0.10-0.17]; no analysis of liver toxicities).
- Johnson TJ, Hedge DD. Esomeprazole: a clinical review. Am J Health Syst Pharm 2002; 59: 1333-9. [PubMed: 12132559](Esomeprazole is the S-isomer of omeprazole and the two are similar in tolerability; no specific discussion of hepatotoxicity).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to an H2 blocker or proton pump inhibitor).
- de Abajo FJ, Montero D, Madurga M, García Rodríguez LA Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol 2004; 58: 71-80. [PMC free article: PMC1884531] [PubMed: 15206996](Analysis of General Practice Research Database from UK on 1.6 million persons from 1994-2000 found 128 cases of drug induced liver injury [2.4/100,000 person years]; 3 cases were attributed to cimetidine for an odds ratio of 2.0 compared to controls [n=5000], which was not statistically significant).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](Survey of all cases of DILI with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002: 103 cases identified as highly probable, probable or possible, one case attributed to ranitidine and one to omeprazole).
- Capitain O, Lortholary A, Abadie-Lacourtoisie S. [Cytolytic hepatitis and esomeprazole during chemotherapy]. Presse Med 2005; 34: 1235-6. French. [PubMed: 16230965](41 year old woman on cyclic chemotherapy for breast cancer received a single dose of esomeprazole and developed abdominal pain and fatigue, and liver tests were abnormal 7 days later [bilirubin normal, ALT 13 times and Alk P 1.5 times ULN], resolving in 8 days).
- El-Matary W, Dalzell M. Omeprazole-induced hepatitis. Pediatr Emerg Care 2005; 21: 529-30. [PubMed: 16096600](15 year old with Crohn disease and acute gastritis developed jaundice 5 days after starting omeprazole [bilirubin 3.2 mg/dL, ALT 218 U/L, Alk P 3018 U/L, INR 1.9], resolving rapidly, all except Alk P being normal within 6 days of stopping).
- Youssef SS, Iskandar SB, Scruggs J, Roy TM. Acute pancreatitis associated with omeprazole. Int J Clin Pharmacol Ther 2005; 43: 558-61. [PubMed: 16372517](83 year old developed symptomatic pancreatitis 2 months after starting omeprazole [amylase 1,137 U/L, lipase 542 U/L], improving promptly on stopping and recurring within 2 days of restarting [amylase 233 U/L, lipase 648 U/L]; no liver test abnormalities mentioned).
- Salgueiro E, Rubio T, Hidalgo A, Manso G. Safety profile of proton pump inhibitors according to the spontaneous reports of suspected adverse reactions. Int J Clin Pharmacol Ther 2006; 44: 548-56. [PubMed: 17176621](Analysis of reports to the Spanish Pharmacovigilance Database during 2004 found 58 reports of liver injury from PPIs including omeprazole [n=36], lansoprazole [7], pantoprazole [12], rabeprazole [2] and esomeprazole [1], correlating somewhat with relative number of prescriptions; 82% were taking other medications; most "evolved to recovery").
- Sánchez Garrido A. [Omeprazole-induced acute cholestatic hepatitis]. Gastroenterol Hepatol 2007; 30: 54. Spanish. [PubMed: 17266883](37 year old developed jaundice 4 days after starting omeprazole [bilirubin 14.2 mg/dL, ALT 852 U/L, Alk P 539 U/L], with resolution in 3 months).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther 2007; 25: 1401-9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury due to drugs between 1993-1999 in Spain; 8 were attributed to ranitidine alone [incidence 5.1/100,000 person years] and 5 to omeprazole alone [2.1/100,000]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 2 were attributed to ranitidine, none to cimetidine or omeprazole).
- Domínguez-Leñero V, Barrera-Ledesma M, Romero-Alonso M, Garrido Martínez MT. [Stevens-Johnson syndrome and toxic hepatitis due to esomeprazole]. Farm Hosp 2009; 33: 118-9. Spanish. [PubMed: 19480805](66 year old with dyspepsia developed fever, diarrhea and severe rash 9 days after switching from pantoprazole to esomeprazole [40 mg/day], evolving to full fledged Stevens Johnson syndrome with hypotension, septic shock, and death from multiorgan [including hepatic] failure; few details given).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](World wide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, there were no antiulcer medications in the top 40 causes).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, none were attributed to omeprazole or other antiulcer medications).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to omeprazole or other proton pump inhibitors).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to antiulcer medications, either H2 blockers or proton pump inhibitors).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 3 [0.3%] were attributed to a proton pump inhibitor, including one each for esomeprazole, omeprazole and lansoprazole).
- van der Schaft J, van Schaik RH, van den Broek MP, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Increased liver enzyme levels during azathioprine treatment: beware of concomitant use of proton pump inhibitors. Br J Dermatol 2015; 173: 1338-9. [PubMed: 26139089](Three patients with atopic dermatitis treated with azathioprine [150 mg daily] developed ALT elevations 2-5 weeks after starting pantoprazole or omeprazole [ALT 14 to 25 U/L before rising to 42 to 254 U/L after], resolving within 1-6 months after stopping the PPI despite continuation of azathioprine).
- Thomas B, Mohamed M, Al Hail M, Awwad FA, Wahba RM, Hassan SB, Omar K, et al. A case of probable esomeprazole-induced transient liver injury in a pregnant woman with hyperemesis. Clin Pharmacol 2016; 8: 199-202. (22 year old woman in first trimester developed pain and nausea after starting metoclopramide and esomeprazole [40 mg twice daily] for hyperemesis gravidarum [bilirubin not provded, ALT 267 U/L, Alk P 69 U/L], resolving rapidly on switching to pantoprazole). [PMC free article: PMC5167460] [PubMed: 28008288]
- Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Let al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients With cirrhosis in a population study. Gastroenterology 2017; 152: 134-41. [PubMed: 27639806](Analysis of the Taiwan National Health Insurance database found a higher rate of PPI use among patients with cirrhosis who developed hepatic encephalopathy [38%: 445 of 1166] compared to a matched group with cirrhosis who did not [rate not provided]; the relative risk was raised for all agents except for rabeprazole).
- Weersink RA, Bouma M, Burger DM, Drenth JPH, Froukje Harkes-Idzinga S, Hunfeld NGM, Metselaar HJ, et al. Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol 2018; 84: 1806-20. [PMC free article: PMC6046475] [PubMed: 29688583](Systematic review of 69 publications in the literature suggested plasma levels of many PPIs are several fold elevated cirrhosis, but less so for omeprazole and esomeprazole but only esomeprazole should be used in patients with Child Pugh Class C cirrhosis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review [New-generation proton pump inhibitors: progress in the treatment of peptic acid diseases?].[Presse Med. 2004]Review [New-generation proton pump inhibitors: progress in the treatment of peptic acid diseases?].de Korwin JD, Ducrotté P, Vallot T. Presse Med. 2004 Jun 19; 33(11):746-54.
- Review Pantoprazole.[LiverTox: Clinical and Researc...]Review Pantoprazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Proton Pump Inhibitors.[LiverTox: Clinical and Researc...]Review Proton Pump Inhibitors.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Meta-analysis of the effectiveness of esomeprazole in gastroesophageal reflux disease and Helicobacter pylori infection.[J Clin Pharm Ther. 2015]Review Meta-analysis of the effectiveness of esomeprazole in gastroesophageal reflux disease and Helicobacter pylori infection.Teng M, Khoo AL, Zhao YJ, Lin L, Lim BP, Wu TS, Dan YY. J Clin Pharm Ther. 2015 Aug; 40(4):368-75. Epub 2015 Apr 20.
- Review Lansoprazole.[LiverTox: Clinical and Researc...]Review Lansoprazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Omeprazole - LiverToxOmeprazole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...