NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Omega-3 fatty acids are essential polyunsaturated fatty acids that have diverse functions in normal metabolism and health and are used as nutritional supplements for general health and for disease prevention and as prescription drugs for treatment of hypertriglyceridemia. The omega-3 fatty acids are generally safe and well tolerated and have not been implicated in causing serum enzyme elevations or clinically apparent liver injury.
Background
The omega-3 fatty acids are essential fatty acids that serve several important functions in normal metabolism and health. Omega-3 refers to their common structural feature of an unsaturated double bond at the third carbon bond from the “omega” end of the long chain fatty acid (n-3). There are three essential omega-3 fatty acids: alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA is an 18-carbon fatty acid with 3 double bonds (18:3n-3) and is found in plant oils such as walnut, flaxseed and canola oil. EPA, a 21-carbon molecule with 5 double bonds (21:5n-3), and DHA, a 22-carbon molecule with 6 double bonds (22:6n-3), are found in marine oils such as fish oils, squid oils and krill oil. Being essential, these fatty acids are not (or poorly) synthesized by humans and the necessary amounts must be provided in the diet. The amounts of omega-3 fatty acids in a typical Western diet can be marginal or inadequate, particularly EPA and DHA in persons with limited fish intake. For these reasons, the omega-3 fatty acids are some of the most commonly used nutrition supplements. They have been proposed to be not only necessary for good health, but also to be effective in prevention of many chronic conditions, including different forms of cancer, coronary artery disease, cerebrovascular disease, developmental disabilities, depression, bipolar illness, cognitive decline, Alzheimer disease, macular degeneration, rheumatoid arthritis, eczema and allergic conditions. However, efficacy of omega-3 fatty acid supplementation in any of these conditions has not been proven and results of prospective controlled trials have been largely negative or at most conflicting. Nevertheless, omega-3 fatty acids are popular nutritional supplements and hundreds of products are available under many commercial names such as “GNC Krill Oil”, “Nordic Natural DHA”, “Carlson Fish Oil Q”, “iHealth Overga-3”, “Jarrow Formulas Flaxseed Oil”, “ProThera Eicosamax Fish”, and “Swanson EFAs”, among others. These products are usually in the form of capsules and vary widely in concentration of the individual omega-3 fatty acids, but are generally in the range of 250 mg to 1,000 mg of total omega-3 fatty acids and recommended as being taken once daily. Side effects of omega-3 fatty acid and fish oil supplements in these doses are minimal, but may include mild gastrointestinal discomfort, nausea, diarrhea and headache. More clinically significant side effects include platelet dysfunction and an increased risk of bleeding, particularly in patients on anticoagulant and antithrombotic therapy.
Hepatotoxicity
In the many, large, randomized controlled trials of the omega-3 fatty acids, side effects have been minimal. Use of omega-3 fatty acids even in high doses has not been linked convincingly to serum enzyme elevations or to instances of clinically apparent liver injury. At high doses used to treat hypertriglyceridemia, minor ALT elevations were identified in up to 12% of patients, but similar rates occurred in placebo treated subjects and the abnormalities were transient, mild and not associated with symptoms or jaundice. Indeed, there have been several clinical trials of various formulations of the omega-3 fatty acids in patients with nonalcoholic fatty liver and preexisting elevations in serum aminotransferase levels. While not demonstrating a convincing beneficial effect in fatty liver disease, the omega-3 fatty acids also did not demonstrate any evidence of hepatic injury or worsening of the preexisting serum enzyme elevations.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Omega-3 fatty acids are metabolized in the liver by beta-oxidation and broken down locally, usually into short chain fatty acids. They have little effect on hepatic cytochrome P450 or drug transporter activity. Their effects on triglyceride metabolism are more likely to be beneficial than harmful to hepatocytes.
Prescription Omega-3 Fatty Acid Preparations
In addition to their use in nutritional supplements, omega-3 fatty acids have been developed as therapies of dyslipidemia and approved as prescription drugs or “medical foods” under the ingredient names omega-3 acid ethyl esters (Lovaza 2004, and generics), icosapent ethyl (Vascepa 2012), and omega-3 carboxylic acids (Epanova 2014). When used to lower triglyceride levels, omega-3 fatty acids are taken in high doses, between 2 and 4 grams daily. These omega-3 fatty acid derivatives are approved as adjunctive therapy of severe hypertriglyceridemia to be used in combination with diet with or without other lipid lowering medications. The mechanism of action of the omega-3 fatty acids in reducing triglyceride levels is not clearly defined, but appears to be inhibition of synthesis of triglycerides in the liver and their accelerated degradation, perhaps through increased plasma lipoprotein lipase activity. These three omega-3 fatty acid derivatives are discussed individually below.
Omega-3 Acid Ethyl Esters
Lovaza (previously known as Omacor) consists of a mixture of omega-3 fatty acid ethyl esters that have been purified from fish oil. The major fatty acid constituents are EPA and DHA, but other long-chain polyunsaturated fatty acid esters are also present in lower concentrations. The starting oil is screened for heavy metal or polychlorinated biphenyl (PCB) contamination and the pooled product purified and esterified to give the final product stability. In preregistration trials in patients with severe hypertriglyceridemia (above 500 mg/dL), Lovaza in doses of 4 grams daily was associated with a 45% decrease in serum triglycerides in comparison to no change or a slight increase with placebo. Therapy was also associated with mild-to-moderate increases low density lipoprotein (LDL) cholesterol. A major complication and reason for treating hypertriglyceridemia is acute pancreatitis, but rates of attacks of pancreatitis could not be evaluated in these trials because of the limited sample size and treatment duration. Lovaza was approved for use in the United States in 2004. Several generic forms are now available, usually as 1 gram capsules. The recommended dose is 4 g daily (in one or two divided doses). Side effects are uncommon and generally mild, but can include dyspepsia, diarrhea, nausea, eructation and unpleasant aftertaste. Potentially more severe adverse events include an increased risk of bleeding, particularly if taken with other anticoagulants or antiplatelet agents.
In preregistration clinical trials, omega-3 acid ethyl esters were associated with uncommon and mild increases in serum ALT levels without changes in alkaline phosphatase or bilirubin levels. These abnormalities were asymptomatic and transient, but led to a recommendation of monitoring routine liver tests periodically during therapy with Lovaza. Since approval and wide scale use of this product, however, there have been no reports of liver injury attributable to the omega-3 acid esters and subsequent trials of these products have not mentioned serum aminotransferase elevations or hepatotoxicity in discussions of adverse events. The product label now recommends monitoring ALT and AST levels periodically only in patients with preexisting hepatic impairment, a recommendation added to product labels of all prescription omega-3 fatty acids.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Icosapent Ethyl
Vascepa is the commercial name for icosapent (eye koe’ sa pent) ethyl, an ethyl ester of eicosapentaenoic acid (EPA) extracted and purified from fish oil. During absorption, icosapent is de-esterified to the active EPA molecule. In preregistration trials, icosapent in doses of 4 grams daily lowered serum triglyceride levels by 27% in patients with severe hypertriglyceridemia (above 500 mg/dL) compared to minor increases with placebo. Rates of pancreatitis could not be evaluated in these trials because of the limited sample size and treatment duration. In contrast to omega-3 fatty acid ethyl esters, icosapent had little effect on serum total or LDL cholesterol levels, perhaps because it consists of modified EPA only without DHA. Vascepa was approved for use in the United Sates in 2012. It is available in 1 gram capsules and the recommended dose is 2 g twice daily. Side effects are uncommon and generally mild, but can include arthralgia, diarrhea, nausea, abdominal pain and eructation. Potential severe adverse events include an increased risk of bleeding, particularly if taken with other anticoagulants or antiplatelet agents.
In preregistration clinical trials in patients with hypertriglyceridemia, liver test abnormalities were no more frequent among patients receiving icosapent than in those on placebo, and there were no reports of clinically apparent liver injury. In a pooled analysis of studies, mild elevations in ALT (up to 2 times the upper limit of normal) occurred in 12.8% of icosapent- compared to 10.3% of placebo treated subjects. However, no patient developed jaundice or symptoms and the abnormalities apparently resolved without dose adjustment. Since its approval and more widespread clinical use, there have been no published reports of hepatotoxicity attributable to icosapent. Nevertheless, the product labels of all prescription omega-3 fatty acid products recommend monitoring aminotransferase levels in patients with preexisting hepatic impairment.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Omega-3 Carboxylic Acids
Epanova is the commercial name of a fish oil extract and mixture of omega-3 carboxylic acids, predominantly derivatives of EPA and DPA. In preregistration clinical trials, Epanova in a dose of 4 g daily lowered serum triglyceride levels by 31% in patients with severe hypertriglyceridemia (above 500 mg/dL) compared to minor changes with placebo. Rates of pancreatitis could not be evaluated in these trials because of the limited sample size and treatment duration. Similar to omega-3 fatty acid ethyl esters, omega-3 carboxylic acid treatment was associated with mild-to-moderate increases in LDL cholesterol levels. Epanova was approved for use in the United Sates in 2014 and is available in 1 gram capsules; the recommended dose being 2 or 4 g once daily. Side effects are generally mild, but can include diarrhea, nausea, abdominal pain and eructation. Potential severe adverse events include an increased risk of bleeding, particularly if taken with other anticoagulants or antiplatelet agents.
In preregistration clinical trials in patients with hypertriglyceridemia, liver test abnormalities were no more frequent among patients receiving omega-3 carboxylic acids than in those on placebo, and there were no reports of clinically apparent liver injury. In a pooled analysis of studies, mild elevations in ALT (up to 2 times the upper limit of normal) occurred in less than 1% of omega-3 carboxylic acid treated subjects and a similar proportion of those on placebo. There were no elevations above 5 times the upper limit of normal and no patient developed jaundice or symptoms and the abnormalities apparently resolved without dose adjustment. Since its approval and more widespread clinical use, there have been no published reports of hepatotoxicity attributable to Epanova. Nevertheless, the product labels of all prescription omega-3 fatty acid products recommend monitoring aminotransferase levels in patients with pre-existing hepatic impairment.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Herbals and Botanical Supplements, Nutritional Supplements; Antilipemic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Omega-3 Fatty Acids – Generic®
Omega-3 Acid Ethyl Esters – Generic, Lovaza®
Icosapent Ethyl – Vascepa®
Omega-3 Carboxylic Acids – Epanova®
DRUG CLASS
Herbal and Botanical Supplements
COMPLETE LABELING
Fact Sheet at National Center for Complementary and Integrative Health, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| ALA | 463-40-1 | C18-H30-O2 |
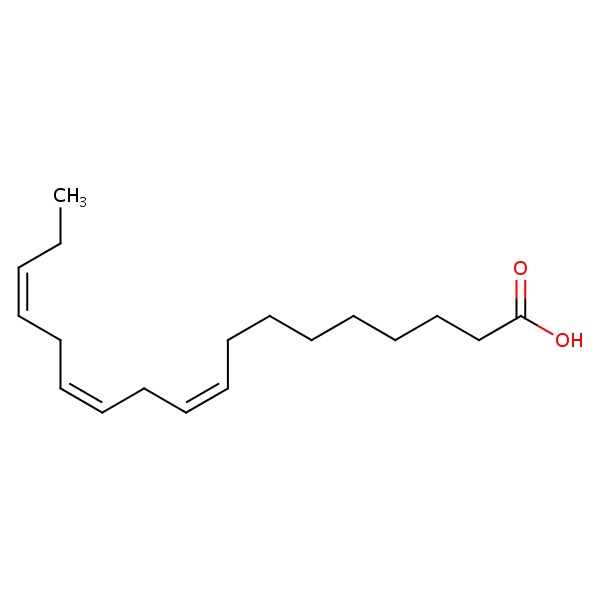 |
| EPA | 1553-41-9 | C20-H30-O2 |
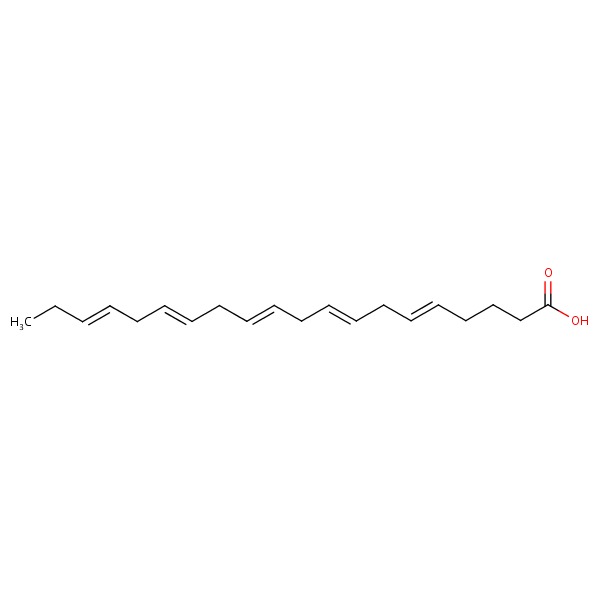 |
| DHA | 25167-62-8 | C22-H32-O2 |
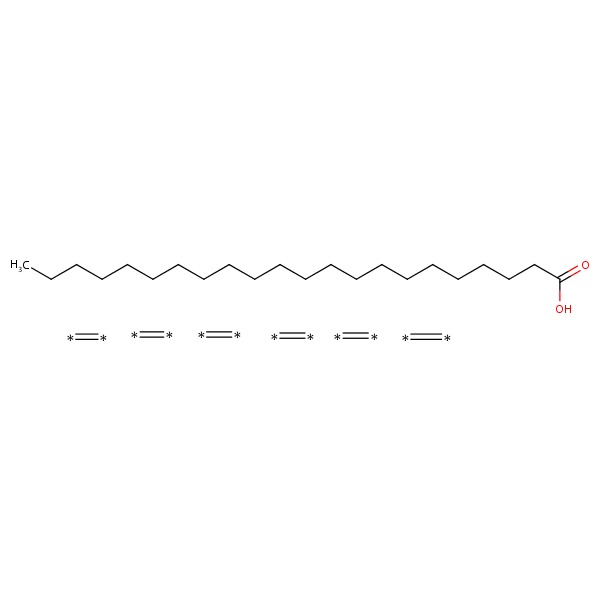 |
ANNOTATED BIBLIOGRAPHY
References updated: 08 April 2017
Abbreviations used: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; the omega-3 fatty acids are not discussed).
- Jones PJH, Kubow S. Lipids, steroids, and their metabolites. In, Shils ME, Olson JA, Shike M, Ross AC, eds. Modern nutrition in health and disease. 9th ed. Baltimore: Williams & Wilkins, 1998; pp. 6794.(Textbook of nutrition).
- Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, Berglund L, Osmundsen K. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk 1997; 4 (5-6): 385-91. [PubMed: 9865671](Among 42 patients with hypertriglyceridemia treated with omega-3 acid ethyl esters [4 g daily] or placebo for 4 months, triglyceride levels decreased by 45% with omega-3 fatty acid therapy vs no effect of placebo and there were no severe adverse events or discontinuations, and therapy had “no impact on any of the safety panel parameters measured, including…liver enzymes”).
- Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, France M. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart 2001; 85: 544-8. [PMC free article: PMC1729738] [PubMed: 11303007](Among 59 patients with hypertriglyceridemia on long term therapy with simvastatin and persistent triglyceride elevations who were treated with omega-3 fatty acids [4 g daily] or placebo for 24 weeks, triglycerides decreased by 30-40% and “there were no apparent differences” between the two groups “in the frequency of laboratory values outside the reference range”).
- Omega-3 polyunsaturated fatty acids(Omacor) for hypertriglyceridemia. Med Lett Drugs Ther 2005; 47 (1221): 91. [PubMed: 16267495](Concise review of mechanism of action, clinical efficacy, adverse effects and costs of omega-3 fatty acid ethyl esters shortly after their approval for use in hypertriglyceridemia; no mention of ALT elevations or hepatotoxicity).
- Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D'Haens G, Bradette M, Cohen A, et al. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA 2008; 299: 1690-7. [PubMed: 18398081](Among 753 patients with Crohn disease in remission treated with omega-3 carboxylic acids [4 g daily] or placebo in two controlled trials, the 1 year relapse rates were similar in both treatment groups in both studies and adverse events were uncommon, those more frequent with Epanova being nausea, diarrhea and dysgeusia, and there were “no differences in laboratory results” identified except for lower triglycerides in omega-3 fatty acid treated subjects).
- Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA 2010; 304: 2363-72. [PubMed: 21078810](Among 663 patients with recurrent atrial fibrillation treated with omega-3 fatty acids [4 g daily] or placebo for 24 weeks, rates of atrial fibrillation recurrence were similar in the 2 groups as were adverse event rates including “abnormal liver tests” [<1% in both groups]).
- Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels(from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 2011; 108: 682-90. [PubMed: 21683321](Among 229 patients with hypertriglyceridemia [>500 mg/dL] treated with icosapent [2 g or 4 g daily] or placebo for 12 weeks, serum triglyceride levels decreased by 20-33% on icosapent vs no change on placebo, while adverse event rates were similar in all groups and neither dose of icosapent produced significant changes in aminotransferase levels).
- Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012; 110 984-92. [PubMed: 22819432](Among 702 patients on statins with persistent triglyceride elevations [200-500 mg/dL] treated with icosapent [2 or 4 g daily] or placebo for 12 weeks, triglyceride levels decreased by 10-22% with icosapent and rates of adverse events were similar in all treatment groups, and no “clinically significant increases” in ALT or AST occurred in icosapent treated groups).
- Fish oil supplements. Med Lett Drugs Ther 2012; 54 (1401): 83-4. [PubMed: 23059421](Concise review of 2 omega-3 fatty acid preparations approved as treatment for severe hypertriglyceridemia, Omacor [Lovaza] and Vascepa; mentions adverse events of dyspepsia, eructation and unpleasant aftertaste, but does not mention ALT elevations or hepatotoxicity).
- Risk and Prevention Study Collaborative Group, Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013; 368: 1800-8. [PubMed: 23656645](Among 12,513 adults with cardiovascular risk factors treated with omega-3 fatty acids or placebo daily for a median of 5 years, there were no significant differences in rates of cardiovascular endpoints or death nor in adverse event rates; no mention of ALT levels or hepatotoxicity).
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013; 309: 2005-15. [PubMed: 23644932](Among 4203 adults [ages 50 year or older] treated with omega-3 fatty acids or lutein with zeaxanthin or both or placebo for a median of 5 years, there were no differences in rates of macular degeneration among treatment groups and adverse event rate were similar; no mention of ALT elevations or hepatotoxicity).
- Icosapent ethyl (Vascepa) for severe hypertriglyceridemia. Med Lett Drugs Ther 2013; 55 (1415): 33-4. [PubMed: 23836343](Concise review of the mechanism of action, clinical efficacy, safety and costs of icosapent ethyl shortly after its approval in the US for treatment of severe hypertriglyceridemia; mentions minor side effects, but not ALT elevations or hepatotoxicity).
- Drugs for hypertriglyceridemia. Med Lett Drugs Ther 2013; 55 (1411): 17-9. [PubMed: 23467119](Concise review of drugs approved for therapy of hypertriglyceridemia including fibrates, niacin and fish oil; mentions risk of liver injury with fibrates and niacin, but not fish oils or omega-3 fatty acids).
- Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, Kling D, et al. Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol 2014; 8: 94-106. [PubMed: 24528690](Among 399 patients with hypertriglyceridemia [>500 mg/dL] treated with Epanova [2, 3 or 4 g daily] or olive oil for 12 weeks, fasting triglyceride levels decreased by 26-31% with omega-3 carboxylic acids vs 4% on olive oil, and adverse events more common with the omega-3 fatty acids included diarrhea [6-10% vs 25], abdominal discomfort [1-4% vs 1%], nausea [5-9% vs 1%] and eructation [3-4% vs 1%]; no mention of ALT elevations or hepatotoxicity).
- Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M; EPE-A Study Group. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014; 147: 377-84. e1. [PubMed: 24818764](Among 243 adults with NAFLD treated with ethyl-eicosapentanoic acid [1800 or 2700 mg] or placebo daily for 12 months, improvements in serum triglycerides but not liver histology or ALT levels were greater among the omega-3 fatty acid treated groups and “there were no significant differences in the rates of any specific or overall adverse events across the study arms”).
- Writing Group for the AREDS2 Research Group., Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, et al. Effect of long-chain ω-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med 2014; 174: 763-71. [PubMed: 24638908](Among 4203 participants in the AREDS2 clinical trial who were treated with omega-3 fatty acids, xanthophylls (or both) or placebo for a median of 4.8 years, there were no differences in rates of cardiovascular outcomes or death or of adverse events by treatment group; no mention of ALT levels and hepatotoxicity).
- Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, Moyses HE, et al.; WELCOME Study. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology 2014; 60: 1211-21. [PubMed: 25043514](Among 103 patients with NAFLD treated with omega-3 fatty acids [4 g daily] or placebo for 15-18 months, there were no significant differences in changes in ALT, AST, and liver or visceral fat between the two groups; no mention of adverse events).
- Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med 2014; 126: 7-18. [PubMed: 25387209](Summary of the three forms of prescription omega-3 fatty acids approved in the US as therapy for severe hypertriglyceridemia).
- Su TC, Hwang JJ, Huang KC, Chiang FT, Chien KL, Wang KY, Charng MJ, et al. A randomized, double-blind, placebo-controlled clinical trial to assess the efficacy and safety of ethyl-ester omega-3 fatty acid in Taiwanese hypertriglyceridemic patients. J Atheroscler Thromb 2017; 24: 275-89. [PMC free article: PMC5383544] [PubMed: 27600795](Among 253 Taiwanese adults with high triglyceride levels [200-1000 mg/dL] treated with omega-3 acid ethyl esters [4 g daily] vs placebo for 8 weeks, triglyceride levels decreased by 30% vs 5%, while adverse events were uncommon and similar between treatment groups; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention.[Nutr Metab Cardiovasc Dis. 2018]Review Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention.Arca M, Borghi C, Pontremoli R, De Ferrari GM, Colivicchi F, Desideri G, Temporelli PL. Nutr Metab Cardiovasc Dis. 2018 Mar; 28(3):197-205. Epub 2017 Nov 13.
- Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials.[J Psychopharmacol. 2021]Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials.Goh KK, Chen CY, Chen CH, Lu ML. J Psychopharmacol. 2021 Mar; 35(3):221-235. Epub 2021 Feb 15.
- Review [Lipids, depression and suicide].[Encephale. 2003]Review [Lipids, depression and suicide].Colin A, Reggers J, Castronovo V, Ansseau M. Encephale. 2003 Jan-Feb; 29(1):49-58.
- Review Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia.[Pharmacotherapy. 2007]Review Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia.McKenney JM, Sica D. Pharmacotherapy. 2007 May; 27(5):715-28.
- Digital Technology Tools to Examine Patient Adherence to a Prescription-Only Omega-3 Polyunsaturated Fatty Acid Therapy To Mitigate Cardiovascular Risk: Protocol for a Prospective Observational Study and Preliminary Demographic Analysis.[JMIR Res Protoc. 2021]Digital Technology Tools to Examine Patient Adherence to a Prescription-Only Omega-3 Polyunsaturated Fatty Acid Therapy To Mitigate Cardiovascular Risk: Protocol for a Prospective Observational Study and Preliminary Demographic Analysis.Arutyunov GP, Arutyunov AG, Ageev FT, Fofanova TV. JMIR Res Protoc. 2021 Aug 30; 10(8):e29061. Epub 2021 Aug 30.
- Omega-3 Fatty Acids - LiverToxOmega-3 Fatty Acids - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...