NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Halothane is a potent volatile halogenated anesthetic gas that has been linked to many cases of idiosyncratic acute liver injury that are frequently severe. The potential of halothane to cause hepatotoxicity and the greater safety of newer anesthetics has led to a decrease in its use, currently limited to special situations, particularly in children. Because halothane is relatively inexpensive it continues to be used in developing countries.
Background
Halothane (hal' oh thane) is a volatile anesthetic that was used widely in major surgery between its introduction in 1956 and falling out of favor in the mid 1990s. It is nonflammable, potent and well tolerated. Halothane is administered to produce end tidal concentrations of 0.7% to 1%. It has a somewhat slow onset of action and, therefore, like other halogenated inhalational anesthetics, it is used to maintain anesthesia after induction with other agents. Halothane is no longer available in the United States, but is still used in developing countries, particularly in pediatric patients. Halothane must be administered in a controlled situation by a properly trained and credentialed anesthesiologist or nurse anesthetist and is typically given in concentrations up to 1% with oxygen.
Hepatotoxicity
Prospective, serial blood testing often demonstrates minor transient elevations in serum aminotransferase levels in the 1 to 2 weeks after major surgery and anesthesia with halothane and other halogenated anesthetics. Appearance of ALT levels above 10 times the upper limit of normal, however, is uncommon and points to significant hepatotoxicity. Clinically apparent, severe hepatic injury from halothane is rare, occurring in ~1/15,000 cases after initial exposure, but in ~1/1,000 cases after repeated exposures. The injury is marked by acute elevations in serum aminotransferase levels (5- to 50-fold) and appearance of jaundice within 2 to 14 days of surgery. There are usually minimal increases in alkaline phosphatase levels. Fever occurs before onset of jaundice in a high proportion of patients and eosinophilia in up to 30%. Rash and arthralgias can also accompany the onset of hepatic injury. The acute liver injury may be self-limited and resolve within 4 to 8 weeks, but can be severe and lead to acute liver failure. A strong risk factor is previous exposure to any of the halogenated anesthetics and particularly a history of halothane hepatitis or unexplained fever and rash after anesthesia with one of these agents. Other risk factors are hypotension, older age, obesity and concurrent use of CYP 2E1 inducers. The differential diagnosis of acute liver injury after surgery and anesthesia is sometimes difficult, and a clinical picture similar to halothane hepatitis can be caused by shock or ischemia, sepsis, other idiosyncratic forms of drug induced liver injury and acute viral or herpes hepatitis. Indeed, many cases of severe liver injury arising soon after surgery and attributed to halothane or other halogenated anesthetics in the literature probably represent liver injury from shock and ischemia. Factors favoring the diagnosis of ischemic hepatitis are rapid onset after surgery, extremely high values for ALT, AST and LDH, and subsequent rapid fall in serum enzymes.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of halothane hepatotoxicity is suspected to be immunoallergic, caused by creation of reactive intermediates of the anesthetic. Approximately 60% to 80% of halothane is eliminated unchanged by the lungs, but a proportion is biotransformed by hepatic microsomal enzyme CYP 2E1 to a trifluoroacetic acid which can be detected in the urine, but which also can trifluoroacetylate hepatic proteins, some of which may be immunogenic and induce cytotoxic reactions. The clinical pattern of injury is suggestive of an allergic hepatitis with rapid onset of injury, fever, eosinophilia and accelerated and more severe injury with reexposure. Patients with halothane hepatitis often have antibodies to trifluoroacetylated proteins. On the other hand, the clinical and histological pattern of halothane hepatic injury also resembles chloroform induced liver damage with centrolobular somewhat bland necrosis, suggesting that toxic intermediates of reductive halothane metabolism may cause the injury by direct injury.
Outcome and Management
Severity ranges from mild and transient aminotransferase elevations without symptoms or other evidence of liver injury, to a self limited symptomatic acute hepatitis-like reaction, to a severe, acute hepatic failure. The severity and prognosis may relate in part of patient age, being more severe in the elderly and both milder and less common in children. Obesity may also be both a predisposing factor and predictor of outcome. Chronic liver injury from repeated halothane exposure has been described in health care workers repeatedly exposed to the agent, but the injury does not appear to lead to a chronic hepatitis if the exposure is terminated. Patients with halothane induced hepatitis should be cautioned against future exposure to a fluorinated hydrocarbon anesthetic such as isoflurane, enflurane, desflurane or sevoflurane.
Drug Class: Halogenated Anesthetics
Other Drugs in the Class: Desflurane, Enflurane, Isoflurane, Sevoflurane
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Halothane – Generic, Fluothane®
DRUG CLASS
Anesthetics, Halogenated
Product labeling at DailyMed, National Library of Medicine, NIH
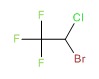
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Halothane | 151-67-7 | C2-H-Br-Cl-F3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 January 2018
- Zimmerman HJ. Anesthetic agents. In, Zimmerman HJ. Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 1999, pp. 457-82.(Review of hepatotoxicity from halothane and other volatile anesthetic agents published in 1999; provides a history of the controversy surrounding halothane hepatotoxicity, along with its biochemical and clinical characteristics, risk factors, histology, and suspected mechanisms).
- Liver disease due to anaesthetic agents. In, Farrell GC. Drug-induced liver disease. Edinburgh: Churchill Livingstone, 1994, pp. 389-412.(Review of the history of halothane hepatitis with overview of the clinical features and pathogenesis).
- Kenna JG. Mechanism, pathology, and clinical presentation of hepatotoxicity of anesthetic agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 403-22.(Review of hepatotoxicity of halothane and other halogenated anesthetic agents focusing on pathogenesis).
- Patel PM, Patel HH, Roth DM. General anesthetics and therapeutic gases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 527-64.(Textbook of pharmacology and therapeutics).
- Brody GL, Sweet RB. Halothane anesthesia as a possible cause of massive hepatic necrosis. Anesthesiology 1963; 24: 29-37. [PubMed: 14015698](Early description of 4 cases of severe liver injury after halothane: 3 women and one man, ages 51-74 years, latency ~12 days to symptoms, 15 days to jaundice, death by 20 days, autopsy showing centrolobular massive necrosis).
- Lindenbaum J, Leifer E. Hepatic necrosis associated with halothane anesthesia. N Engl J Med 1963; 268: 525-30. [PubMed: 13930795](Early, classic clinical description of 8 cases of halothane and one of methoxyflurane hepatitis: fever and then jaundice, typical hepatocellular enzyme pattern, slow to resolve).
- Slater EM, Gibson JM, Dykes MH, Walzer SG. Postoperative hepatic necrosis. Its incidence and diagnostic value in association with the administration of halothane. N Engl J Med 1964; 270: 983-7. [PubMed: 14122794](Among 14,685 procedures done at one institution under halothane anesthesia over a 3 year period, authors found only one case of severe acute hepatic necrosis suggestive of halothane hepatitis).
- Summary of the National Halothane Study. Possible association between halothane anesthesia and postoperative hepatic necrosis. JAMA 1966; 197: 775-88. [PubMed: 5953371](Analysis of all necropsies showing massive hepatic necrosis after anesthesia from 34 institutions from 1959-62 found most cases were explained by ischemia, sepsis or other conditions; among 82 cases, only 9 were considered “unexplained”, 7 followed halothane; Committee recommended further study rather than limitation of halothane use).
- Klein NC, Jeffries GH. Hepatotoxicity after methoxyflurane administration. JAMA 1966; 197: 1037-9. [PubMed: 5953207](58 year old woman developed fever followed by jaundice on day 7 after methoxyflurane anesthesia [bilirubin 4.4 mg/dL, ALT 2000 U/L, Alk P 3.5 time ULN, 28% eosinophils], resolving within one month).
- Babior BM, Davidson CS. Postoperative massive liver necrosis. A clinical and pathological study. N Engl J Med 1967; 276: 645-52. [PubMed: 6018455](Separate pathological review of cases of National Halothane Study; 71 cases with massive necrosis fell into two clinical and histological patterns: 56 vascular [mild or no jaundice, rapid onset, history of heart disease or shock] vs 15 hepatic [usually jaundiced, 3-20 day latency, rapid demise, no shock, architectural disturbance on biopsy]).
- Gall EA. Report of the pathology panel. National Halothane Study. Anesthesiology 1968; 29: 233-48. [PubMed: 5639587](Report of 10,171 autopsies and 197 cases of severe hepatic necrosis; only 19 cases were unexplained, but they had no characteristic histologic pattern different from explained cases).
- Klatskin G, Kimberg DV. Recurrent hepatitis attributable to halothane sensitization in an anesthetist. N Engl J Med 1969; 280: 515-22. [PubMed: 5764450](Famous paper on an anesthesiologist who appeared to suffer multiple relapses of liver disease due to halothane exposure: careful rechallenge was followed with ALT rising from normal to 400 U/L in one day; report predated tests for hepatitis B and C, but liver histology resembled chronic hepatitis C).
- Klion FM, Schaffner F, Popper H. Hepatitis after exposure to halothane. Ann Intern Med 1969; 71: 467-77. [PubMed: 5809678](Analysis of 42 cases of halothane jaundice: 67% fatal; 72% previously exposed; 88% had fever, 95% jaundiced: onset jaundice in 2-15 days, ALT usually >5 times and Alk P <4 times ULN. Histology showed acute hepatitis with focal or submassive necrosis, occasionally steatosis).
- Peters RL, Edmondson HA, Reynolds TB, Meister JC, Curpey TJ. Hepatic necrosis associated with halothane anesthesia. Am J Med 1969; 47: 748-64. [PubMed: 5352203](Analysis of 33 fatal and 8 nonfatal cases of suspected halothane hepatitis referred to pathologists at USC, found obesity and previous exposure to halothane within previous month to be risk factors, clinical pattern of fever several days before jaundice; histology separated into three stages: early, intermediate and late: necrotic, resorptive, and regenerative).
- Hughes M, Powell LW. Recurrent hepatitis in patients receiving multiple halothane anesthetics for radium treatment of carcinoma of the cervix uteri. Gastroenterology 1970; 58: 790-7. [PubMed: 5423891](Six dramatic cases of halothane hepatitis in patients receiving multiple exposures in a protocol of repeated cervical radium implants [2-4] over a short period of time, often with an earlier episode of fever, fatal in 2 cases: no further cases occurred once halothane use was stopped).
- Carney FM, Van Dyke RA. Halothane hepatitis: a critical review. Anesth Analg 1972; 51: 135-60. [PubMed: 4399967](Review of differential diagnosis of liver disease after surgery and anesthesia concluded that “halothane hepatitis is a real but rare entity”).
- Paull A, Grant AK. Halothane hepatitis--a report of five cases. Med J Aust 1974; 1: 954-7. [PubMed: 4852220](Five cases of halothane hepatitis from Australia, latency of 5 to 12 days often preceded by fever occurring after 2 to 4 exposures; 1 fatal, 2 had eosinophilia, AST 100-2800 U/L, bilirubin 3.8-34 mg/L, biopsies showed hepatitis with variable amounts of necrosis, focal to submassive).
- Moult PJ, Sherlock S. Halothane-related hepatitis. A clinical study of twenty-six cases. Q J Med 1975; 44: 99-114. [PubMed: 1153692](Classic review of halothane hepatitis; 26 patients, 25 adults, 69% women, often obese, 92% previously exposed, symptom onset in 1-12 days, jaundice rapidly following, AST markedly elevated, Alk P minimally increased, bilirubin 3.2-41.2 mg/dL; 42% fatal, no chronicity; striking demonstration of fever with previous exposures and acceleration of latency).
- Wright R, Eade OE, Chisholm M, Hawksley M, Lloyd B, Moles TM, Edwards JC, Gardner MJl. Controlled prospective study of the effect on liver function of multiple exposures to halothane. Lancet 1975; 1: 817-20. [PubMed: 48053](Prospective study randomizing to halothane anesthesia or not and following AST at regular intervals found elevations in 15 halothane exposed and 4 control patients; peak AST levels 40-440 U/L ~7 days, all self limiting and asymptomatic; one case had recurrence on reexposure).
- Hoft RH, Bunker JP, Goodman HI, Gregory PB. Halothane hepatitis in three pairs of closely related women. N Engl J Med 1981; 304: 1023-4. [PubMed: 7207555](3 pairs of cases of halothane hepatitis in close relatives: mother-daughter, sisters, first cousins; all Mexican, onset 2-10 days after anesthesia with fever [5/6], eosinophilia [3/6], half of which were fatal [3/6], somewhat vague in details).
- Neuberger J, Mieli-Vergani G, Tredger JM, Davis M, Williams R. Oxidative metabolism of halothane in the production of altered hepatocyte membrane antigens in acute halothane-induced hepatic necrosis. Gut 1981; 22: 669-72. [PMC free article: PMC1420052] [PubMed: 7286784](Serum from halothane hepatitis cases was cytotoxic to hepatocytes from rabbits given halothane, but only if animals were exposed to high concentrations of oxygen suggesting that oxidative metabolic pathway was involved).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol 1982; 17: 205-11. [PubMed: 6982502](Analysis of 572 cases of drug induced liver disease reported to the Danish Board on Adverse Reactions to Drugs between 1968-78 revealed halothane to be the most commonly incriminated agent in causing liver injury, 143 cases [25% of total number]).
- Benjamin SB, Goodman ZD, Ishak KG, Zimmerman HJ, Irey NS. The morphologic spectrum of halothane-induced hepatic injury: analysis of 77 cases. Hepatology 1985; 5: 1163-71. [PubMed: 4065822](77 cases of halothane hepatotoxicity from files of Armed Forces Institute of Pathology; mean age was 50 years, 62% women, 46% obese, 73% previously exposed, symptoms arising 1-13 days after anesthesia, marked ALT elevations, 56% fatal; careful depiction of histology marked by centrolobular submassive or spotty necrosis and portal inflammation with eosinophils and neutrophils).
- Pohl LR, Kenna JG, Satoh H, Christ D, Martin JL. Neoantigens associated with halothane hepatitis. Drug Metab Rev 1989; 20: 203-17. [PubMed: 2680380](Review of work on pathogenesis of halothane hepatitis, characterizing neoantigens covalently modified by tifluoroacetate [TFA] under aerobic conditions; also found after isoflurane and enflurane exposure).
- Martin JL, Kenna JG, Pohl LR. Antibody assays for the detection of patients sensitized to halothane. Anesth Analg 1990; 70: 154-9. [PubMed: 2301746](ELISA assays for anti-TFA using rat serum albumin had a high false positive rate and poor sensitivity compared to ELISAs and immunoblotting using purified TFA microsomal proteins as test antigens).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med 1992; 232: 133-8. [PubMed: 1506809](In late 1980s, halothane was still the most common cause of drug induced liver injury in Denmark, but decreasing in frequency).
- Martin JL, Reed GF, Pohl LR. Association of anti-58 kDa endoplasmic reticulum antibodies with halothane hepatitis. Biochem Pharmacol 1993; 46: 1247-50. [PubMed: 8216376](Patients with halothane hepatitis develop antibody to a 58 kDa endoplasmic reticulum [ER] protein that becomes trifluoroacetylated with halothane exposure).
- Bourdi M, Chen W, Peter RM, Martin JL, Buters JTM, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol 1996; 9: 1159-66. [PubMed: 8902272](Anti-CYP 2E1 found in serum of 25 of 56 patients with halothane hepatitis; antibody inhibited the enzyme and was directed at conformational epitopes).
- Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol 1996; 50: 573-82. [PubMed: 8794896](Rats treated with halothane developed trifluoroacetylated CYP2E1 in liver; and same protein is recognized by antibody present in 14 of 20 patients with halothane hepatitis, rarely in controls; TFA protein possibly present on hepatocyte membranes).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J 1996; 109: 315-9. [PubMed: 8816722](Spontaneous reporting of 943 cases of drug induced liver injury from 1974-94 in New Zealand implicated 205 different drugs; halothane ranked #2 [n=64] overall, but there was a decrease in frequency over the 21 years, ranking #7 from 1988-94).
- Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, Pohl LR. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth Analg 2002; 94: 243-9, table of contents. [PubMed: 11812677](Anti-CYP 2E1 found in anesthesiologists exposed to halothane; no apparent adverse effects).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 3 were attributed to halothane and 1 to isoflurane, but none to other halogenated anesthetics).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 16 were attributed to halothane [ranking #1], but none to other halogenated anesthetics).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al.; Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Among 446 cases of drug induced liver disease reported in Spain between 1984-2004, neither halothane nor other anesthetic agents were listed among the major drugs implicated).
- Martin JL. [Volatile anesthetics and liver injury: a clinical update or what every anesthesiologist should know.] Can J Anaesth 2005; 52: 125-9. English, French. [PubMed: 15684249](Brief review of clinical features and pathogenesis of anesthetic induced liver injury).
- Kumar GP, Bhat VJ, Sowdi V. Fulminant hepatic failure following halothane anaesthesia. J Clin Forensic Med 2005; 12: 271-3. [PubMed: 16085447](38 year old man from Mangalore India underwent 6 operations with halothane anesthesia over 18 days for traumatic wounds and developed jaundice 3 days after last operation and died 5 days later with massive necrosis on autopsy).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. [PubMed: 16054882](Among 4690 reports of fatal drug induced liver injury reported to the WHO database between 1968-2003, halothane ranked #5 with ~85 cases, but ~90% were reported before 1990).
- Njoku DB, Mellerson JL, Talor MV, Kerr DR, Faraday NR, Outschoorn I, Rose NR. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin Vaccine Immunol 2006; 13: 258-65. [PMC free article: PMC1391926] [PubMed: 16467335](Patients with anesthetic induced hepatitis had higher levels of antibodies to CYP 2E1 TFA adducts than controls; IgG4 specific anti-CYP2E1 was more common in cases than controls).
- Qureshi MA, Saeed F, Hussain T. Halothane induced fulminant hepatic failure. J Coll Physicians Surg Pak 2007; 17: 103-4. (A 22 year old Pakistani man developed jaundice 2 days after halothane anesthesia [bilirubin 8.1 rising to 21.3 mg/dL, ALT 645 to 3208 U/L, Alk P 371 to 474 U/L], dying by postoperative day 6). [PubMed: 17288858]
- Eghtesadi-Araghi P, Sohrabpour A, Vahedi H, Saberi-Firoozi M. Halothane hepatitis in Iran: a review of 59 cases. World J Gastroenterol. 2008; 14: 5322-6. [PMC free article: PMC2744064] [PubMed: 18785286](59 cases of halothane hepatitis seen at 7 hospitals in Iran between 1994 and 2006; mean age 44 years, mean time to onset 15 days, 81% women, 22% obese, 61% previous exposure, 6 deaths [12%]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease collected in the US between 2003 and 2008, 2 cases were attributed to desflurane, 1 to sevoflurane, but none to halothane; details not given).
- Björnsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol 2009; 50: 511-7. [PubMed: 19155082](Among 685 patients with drug induced liver disease, 27 had evidence of chronic liver injury during an average of 10 years of following, including a 31 year old woman who had mild halothane hepatitis [bilirubin peak 4.5 mg/dL] and was subsequently found to have an inactive, compensated cirrhosis and died of suicide 11 years later).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. Multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including one due to halothane and one to isoflurane, but none to other anesthetic agents).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. . [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, no anesthetic agents ranked in the top 40 causes)
- Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepat Mon 2011; 11: 3-6. [PMC free article: PMC3206652] [PubMed: 22087107](Review of halothane induced liver injury and the risk factors that might be used to as contraindications in countries where it is still used).
- Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Hepatotoxicity of halogenated inhalational anesthetics. Iran Red Crescent Med J 2014; 16: e20153. [PMC free article: PMC4270648] [PubMed: 25593732](Review of hepatotoxicity of the volatile halogenated anesthetics).
- Lin J, Moore D, Hockey B, Di Lernia R, Gorelik A, Liew D, Nicoll A. Drug-induced hepatotoxicity: incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int 2014; 34: 576-82. [PubMed: 23944929](Retrospective analysis of records from 1556 patients admitted to a trauma unit during 2008 identified 47 cases of possible volatile anesthetic related liver injury, including 12 who developed ALT levels above 200 U/L of whom 11 had fever and 6 eosinophilia, but none were jaundiced or developed acute liver failure).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. (Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases of which 5 [3%] were attributed to halothane and caused acute liver failure, while. [PubMed: 24552865]no other volatile anesthetic was listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 2 were attributed to isoflurane, 1 to sevoflurane, but none to halothane, enflurane or desflurane).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Halogenated Anesthetics.[LiverTox: Clinical and Researc...]Review Halogenated Anesthetics.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis.[Hepat Mon. 2011]Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis.Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Hepat Mon. 2011 Jan; 11(1):3-6.
- Review Hepatotoxicity of halogenated inhalational anesthetics.[Iran Red Crescent Med J. 2014]Review Hepatotoxicity of halogenated inhalational anesthetics.Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Iran Red Crescent Med J. 2014 Sep; 16(9):e20153. Epub 2014 Sep 5.
- Review Anesthetics, General.[LiverTox: Clinical and Researc...]Review Anesthetics, General.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- A mouse model of severe halothane hepatitis based on human risk factors.[J Pharmacol Exp Ther. 2010]A mouse model of severe halothane hepatitis based on human risk factors.Dugan CM, MacDonald AE, Roth RA, Ganey PE. J Pharmacol Exp Ther. 2010 May; 333(2):364-72. Epub 2010 Feb 2.
- Halothane - LiverToxHalothane - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...