NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Desflurane is one of the most commonly used volatile anesthetic agents and has an excellent safety record. Rare single case reports of severe acute liver injury resembling halothane hepatitis due to desflurane have been published.
Background
Desflurane (des flur' ane) is a widely used major anesthetic agent with rapid onset of action and rapid dispersal. Because it can be irritating to the airway, desflurane is typically used to maintain anesthesia after induction with other agents. Desflurane was initially approved for use in the United States in 1992 and is available generically and under the brand name Suprane. Desflurane must be administered in a controlled situation by a properly trained and credentialed anesthesiologist or nurse anesthetist and is typically given in concentrations up to 6% to 8% with oxygen.
Hepatotoxicity
Prospective, serial blood testing often demonstrates minor transient elevations in serum aminotransferase levels in the 1 to 2 weeks after major surgery and use of halogenated anesthetics. Appearance of ALT levels above 10 times the upper limit of normal, however, is distinctly unusual and points to significant hepatotoxicity. Clinically apparent, severe hepatic injury from desflurane is very rare, with only isolated case reports having been published and not all of which were very convincing. The injury is marked by acute elevations in serum aminotransferase levels (5- to 50-fold) and appearance of jaundice within 2 to 21 days of surgery. There are usually minimal increases in alkaline phosphatase and gammaglutamyl transpeptidase levels. Jaundice is usually preceded by a day or two of fever and may be accompanied by rash and eosinophilia. The acute liver injury may be self-limited and resolve within 4 to 8 weeks, but can be severe and associated with acute liver failure. A strong risk factor is previous exposure to any of the halogenated anesthetics and particularly a history of halothane hepatitis or unexplained fever and rash after anesthesia with one of these agents. The differential diagnosis of acute liver injury after surgery and anesthesia is often challenging, and a clinical picture similar to desflurane induced hepatitis can be caused by shock or ischemia, sepsis, acetaminophen overdose, acute viral or herpes hepatitis, as well as other idiosyncratic forms of drug induced liver injury.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of desflurane hepatotoxicity is suspected to be similar to that of halothane and associated with creation of reactive intermediates of desflurane. Desflurane is metabolized to a small but variable extent by the microsomal drug metabolizing enzyme CYP 2E1 to a trifluoroacetylated reactive intermediate (TFA) that is capable of binding to multiple intracytoplasmic proteins, forming potentially immunogenic adducts. The TFA adducts induce antibodies that can be detected in patients with desflurane as well as halothane hepatotoxicity and are also found in a proportion of health care workers exposed to the volatile anesthetics.
Outcome and Management
Severity ranges from mild and transient aminotransferase elevations without symptoms or other evidence of liver injury, to a self limited symptomatic acute hepatitis-like reaction to a severe, acute hepatic failure. The severity and prognosis may relate in part of patient age, being more severe in the elderly and both milder and less common in children. Obesity may also be both a predisposing factor and predictor of outcome. Chronic liver injury from desflurane exposure has not been described. Patients with desflurane induced hepatitis should be cautioned against future exposure to a fluorinated hydrocarbon anesthetic such as halothane, enflurane, isoflurane or sevoflurane.
Drug Class: Halogenated Anesthetics
Other Drugs in the Class: Enflurane, Halothane, Isoflurane, Sevoflurane
CASE REPORT
Case 1. Acute liver injury from desflurane anesthesia.
[Modified from: Tung D, Yoshida EM, Wang CS, Steinbrecher UP. Severe desflurane hepatotoxicity after colon surgery in an elderly patient. Can J Anaesth 2005; 52: 133-6. PubMed Citation]
An 81 year old overweight woman underwent a right hemicolectomy for colon cancer under desflurane anesthesia and developed clinical evidence of liver disease 6 days later. She had a history of two previous surgeries, but the anesthetics used were not known. She had no history of liver disease or known risk factors or exposures. On postoperative day 6, she became symptomatic with fatigue and was jaundiced, but afebrile. Laboratory testing showed a serum bilirubin of 6.9 mg/dL and marked elevations of ALT with minimal increases in alkaline phosphatase (Table). She had no eosinophilia. She was negative for markers of hepatitis A, B and C and autoantibodies, and liver ultrasound was unrevealing. Over the next several days, she developed evidence of hepatic failure with mild hepatic encephalopathy and INR rising to 2.3. She was treated with methylprednisone (60 mg IV daily for 5 days). She began to recover and laboratory tests had largely returned to close to normal by the time she was discharged 7 weeks after surgery.
Key Points
| Medication: | Desflurane (6%), 90 min anesthesia time |
|---|---|
| Pattern: | Hepatocellular (R=>30) |
| Severity: | 4+ (evidence of acute liver failure, INR>1.5) |
| Latency: | 6 days |
| Recovery: | 8-12 weeks |
| Other medications: | Anesthesia included fentanyl, propofol and rocuronium |
Laboratory Values
| Time after Surgery | ALT (U/L) | Alk P (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|
| Pre | 15 | 72 | ||
| 0 | Right hemicolectomy under desflurane anesthesia | |||
| 6 days | 2188 | 149 | 6.9 | Symptoms and jaundice |
| 8 days | 905 | 231 | 9.8 | |
| 10 days | 567 | 296 | ||
| 12 days | 277 | 12.5 | Methylprednisone started | |
| 14 days | 250 | 252 | ||
| 16 days | 200 | 278 | 6.4 | |
| 18 days | 189 | 335 | 5.8 | |
| 20 days | 153 | 316 | 6.8 | |
| 34 days | 45 | 2.4 | ||
| Normal Values | <65 | <200 | <1.2 | |
*Calculated from µmol/L.
Comment
This patient had typical halogenated anesthetic associated acute liver injury with a severe course, but ultimate recovery. Fever and eosinophilia are common at the onset of anesthetic induced hepatitis, but are not invariably present. In this patient and others, prednisone was used, but its benefit in this situation has not been proven. Risk factors for halogenated anesthetic hepatotoxicity included older age, overweight and possible previous exposures to halogenated anesthetics.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Desflurane – Generic, Suprane®
DRUG CLASS
Anesthetics, Halogenated
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
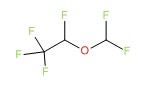
| Desflurane | 57041-67-5 | C3-H2-F6-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 January 2018
- Zimmerman HJ. Anesthetic agents. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 457-82.(Expert review of hepatotoxicity of anesthetic agents published in 1999; mentions that the newer halogenated anesthetics appear to be safer than halothane but that hints of liver injury from desflurane and sevoflurane have appeared).
- Kenna JG. Mechanism, pathology, and clinical presentation of hepatoxicity of anesthetic agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 403-22.(Review of liver injury from anesthetic agents mentions that a few cases of postoperative liver injury have occurred in patients exposed to desflurane).
- Patel PM, Patel HH, Roth DM. General anesthetics and therapeutic gases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 527-64.(Textbook of pharmacology and therapeutics)
- Weiskopf RB, Eger EI 2nd, Ionescu P, Yasuda N, Cahalan MK, Freire B, Peterson N, et al. Desflurane does not produce hepatic or renal injury in human volunteers. Anesth Analg 1992; 74: 570-4. [PubMed: 1554124](Prospective study of 13 young patients given ~7 hours of desflurane anesthesia, with no abnormalities in ALT and AST found during postoperative period).
- Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology 1993; 79: 795-807. [PubMed: 8214760](Human microsomes used to assess metabolism of sevoflurane: CYP 2E1 was major if not sole enzyme catalyzing defluorination of sevoflurane and others).
- Zaleski L, Abello D, Gold MI. Desflurane versus isoflurane in patients with chronic hepatic and renal disease. Anesth Analg 1993; 76: 353-6. [PubMed: 8424515](Prospective study of 20 patients with renal and 20 with hepatic disease given desflurane or isoflurane found no worsening of ALT, AST, Alk P, bilirubin or creatinine).
- Katz J, Magee J, Baker B, Eger EI 2nd. Hepatic necrosis associated with herpesvirus after anesthesia with desflurane and nitrous oxide. Anesth Analg 1994; 78: 1173-6. [PubMed: 8198278](76 year old woman developed fever on 6 days and abdominal pain and diarrhea 11 days after desflurance anesthesia [AST rising to 8000 U/L without jaundice] and death on day 14, autopsy showing massive hepatic necrosis due to herpes hepatitis, rather than anesthetic induced liver injury).
- Martin JL, Plevak DJ, Flannery KD, Charlton M, Poerucha JJ, Humphreys CE, Derfus G, Pohl L. Hepatotoxicity after desflurane anesthesia. Anesthesiology 1995; 83: 1125-9. [PubMed: 7486167](65 year old woman devleloped fever, rash and arthralgias 12 days after desflurane anesthesia [bilirubin 20.3 rising to 30 mg/dL, ALT 1886 U/L, Alk P 202 U/L], referred for liver transplant but recovered spontaneously; had anti-TFA-modified liver microsomal proteins).
- Njoku D, Laster MJ, Gong DH, Eger EI 2nd, Reed GF, Martin JL. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth Analg 1997; 84: 173-8. [PubMed: 8989020](Rats exposed to anesthetics had TFA labeled proteins in hepatocytes: halothane>>enflurane>>isoflurane, but not with desflurane; sera from cases of halothane hepatitis reacted with microsomes from halothane treated rats, but to a lesser extent with desflurane-, enflurane- and isoflurane treated rats).
- Berghaus TM, Baron A, Geier A, Lamerz R, Paumgartner G. Hepatotoxicity following desflurane anesthesia. Hepatology 1999; 29: 613-4. [PubMed: 10026031](37 year old woman developed hepatitis 12 days after desflurane anesthesia [bilirubin 29.4 mg/dL, ALT 1776 U/L, Alk P 185 U/L] with evidence of liver failure, but ultimate recovery; had previous history of probable isoflurane hepatitis; anti-TFA present transiently).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 3 were attributed to halothane and 1 to isoflurane, none to other anesthetics).
- Tung D, Yoshida EM, Wang CS, Steinbrecher UP. Severe desflurane hepatotoxicity after colon surgery in an elderly patient. Can J Anaesth 2005; 52: 133-6. [PubMed: 15684251](81 year old woman developed severe hepatocellular injury 6 days after desflurane anesthesia [bilirubin 6.9 rising to 12.5 mg/dL, ALT 2188 U/L, Alk P 149 U/L], with ultimate full recovery. Case 1).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 16 were attributed to halothane [ranking #1] but none to other anesthetics).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. [PubMed: 16054882](In WHO database of fatal adverse drug reactions from 1968-2003, there were 4690 reports of fatalities due to drug induced liver injury: halothane ranked fifth in frequency as a cause but most cases were reported before 1991).
- Anderson JS, Rose NR, Martin JL, Eger EI, Njoku DB. Desflurane hepatitis associated with hapten and autoantigen-specific IgG4 antibodies. Anesth Analg 2007; 104: 1452-3, table of contents. (22 year old woman developed fever, jaundice and pruritus 17 to 21 days after desflurane anesthesia [bilirubin 6.2 mg/dL, ALT 347 U/L, Alk P 376 U/L] with rapid recovery; had serum antibodies to TFA and CYP 2E1). [PMC free article: PMC3650136] [PubMed: 17513640]
- Côté G, Bouchard S. Hepatotoxicity after desflurane anesthesia in a 15 month old child with Mobius syndrome after previous exposure to isoflurane. Anesthesiology 2007; 107: 843-5. [PubMed: 18073559](15 month old boy developed ALT of 6,180 U/L with INR of 2.7, on postoperative day 3, after desflurane anesthesia [having two previous exposures to isoflurane], with rapid recovery; bilirubin not mentioned and patient also received acetaminophen).
- Her C. Acetaminophen-induced, not desflurane-induced, hepatotoxicity. Anesthesiology 2008; 109: 570-1. [PubMed: 18719456](Letter in response to Côté and Bouchard [2007] suggesting that the liver injury was due to acetaminophen rather than desflurane using Rumack-Matthew nomogram and in view of the high ALT levels).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States.Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease collected in the US between 2003 and 2008, two cases were attributed to desflurane and one to sevoflurane; details not given).
- Chin MW, Njoku DB, MacQuillan G, Cheng WS, Kontorinis N. Desflurane-induced acute liver failure. Med J Aust 2008; 189: 293-4. [PubMed: 18759731](53 year old woman developed ALT elevation to 943 U/L after initial surgery and desflurane exposure and acute liver failure after the second surgery and exposure [bilirubin not given, ALT 11,600 U/L, INR 3.7, anti-TFA positive], autopsy showing massive necrosis).
- Arslan M, Kurtipek O, Dogan AT, Unal Y, Kizil Y, Nurlu N, Kamaci S, et al. Comparison of effects of anaesthesia with desflurane and enflurane on liver function. Singapore Med J 2009; 50: 73-7. [PubMed: 19224088](Prospective study of 40 patients receiving either desflurane or enflurane anesthesia for ALT, AST and glutathione-S-transferase [GST] levels showing elevations in GST but not ALT or AST at 1 hour, 1 and 7 days in enflurane group).
- D.evarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396-404. PubMed Citation (313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India: none were attributed to anesthetic agents].
- Toprak HI, Şahin T, Aslan S, Karahan K, Şanli M, Ersoy MÖ. Effects of desflurane and isoflurane on hepatic and renal functions and coagulation profile during donor hepatectomy. Transplant Proc 2012; 44: 1635-9. [PubMed: 22841233](Serial liver tests after right lobe, living donor hepatectomy showed marked elevations [up to 200 times ULN] in ALT and AST by the end of surgery through day 7, resolving by day 30, and increases in INR [1.1 to 1.5] from the end of surgery to day 5, elevations being greater among 40 donors given isoflurane than 40 given desflurane).
- Iorio ML, Cheerharan M, Kaufman SS, Reece-Stremtan S, Boyajian M. Acute Liver Failure Following Cleft Palate Repair: A Case of Therapeutic Acetaminophen Toxicity. Cleft Palate Craniofac J 2013; 50: 747-50. [PubMed: 22937760](8 month old boy developed lethargy and seizures 3 days after cleft palate repair under desflurance anesthesia and while receiving acetaminophen for pain [bilirubin 3.1 mg/dL, ALT 12,885 U/L], resolving rapidly after NAC therapy).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. (In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to anesthetic agents). [PubMed: 23419359]
- Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Hepatotoxicity of halogenated inhalational anesthetics. Iran Red Crescent Med J 2014; 16: e20153. [PMC free article: PMC4270648] [PubMed: 25593732](Review of hepatotoxicity of the volatile halogenated anesthetics).
- Batistaki C, Michalopoulos G, Matsota P, Nomikos T, Kalimeris K, Riga M, Nakou M, Kostopanagiotou G. CYP2E1 immunoglobulin G4 subclass antibodies after desflurane anesthesia. World J Hepatol 2014; 6: 340-6. [PMC free article: PMC4033291] [PubMed: 24868327](Testing of 39 patients for liver biochemical tests and for IgG4 antibody to CYP2E1 before and 4 days after anethesia with desflurane showed no de novo production or rise in titers of the anti-CYP antibody and no evidence of liver injury).
- Lin J, Moore D, Hockey B, Di Lernia R, Gorelik A, Liew D, Nicoll A. Drug-induced hepatotoxicity: incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int 2014; 34: 576-82. [PubMed: 23944929](Retrospective analysis of records from 1556 patients admitted to a trauma unit during 2008 identified 47 cases of possible volatile anesthetic related liver injury, including 12 who developed ALT levels above 200 U/L of whom 11 had fever and 6 eosinophilia, but none were jaundiced or developed acute liver failure).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. (Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases of which 5 [3%] were attributed to halothane and caused acute liver failure, while. [PubMed: 24552865]no other volatile anesthetic was listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 2 were attributed to isoflurane, 1 to sevoflurane, but none to halothane, enflurane or desflurane).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Halogenated Anesthetics.[LiverTox: Clinical and Researc...]Review Halogenated Anesthetics.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury.[Anesth Analg. 1997]Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury.Njoku D, Laster MJ, Gong DH, Eger EI 2nd, Reed GF, Martin JL. Anesth Analg. 1997 Jan; 84(1):173-8.
- Percutaneous loss of desflurane, isoflurane, and halothane in humans.[Anesthesiology. 1991]Percutaneous loss of desflurane, isoflurane, and halothane in humans.Fassoulaki A, Lockhart SH, Freire BA, Yasuda N, Eger EI 2nd, Weiskopf RB, Johnson BH. Anesthesiology. 1991 Mar; 74(3):479-83.
- Review Sevoflurane.[LiverTox: Clinical and Researc...]Review Sevoflurane.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Enflurane.[LiverTox: Clinical and Researc...]Review Enflurane.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Desflurane - LiverToxDesflurane - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...