NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Doravirine is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents in the therapy of human immunodeficiency virus (HIV) infection. Doravirine is associated with a low rate of transient serum aminotransferase elevations during therapy but has not been implicated in cases of clinically apparent acute liver injury.
Background
Doravirine (dor" a vir' een) is an antiretroviral agent that acts by noncompetitive binding to, and inhibition of, the HIV reverse transcriptase. Doravirine is a nonnucleoside reverse transcriptase inhibitor similar in action to nevirapine, efavirenz, delavirdine, etravirine and rilpivirine. In several randomized controlled trials, doravirine was found to have similar efficacy to efavirenz when combined with at least two nucleoside analogues (typically tenofovir and emtricitabine or lamivudine). Doravirine was the sixth nonnucleoside reverse transcriptase inhibitor approved by the FDA [2018], and current indications are for the treatment of HIV infection in combination with other HIV medications. Doravirine is available under the brand name Pifeltro in tablets of 100 mg. The recommended dose is 100 mg orally once daily. Doravirine is also available in a fixed dose (100 mg), oral once-a-day combination with lamivudine (300 mg) and tenofovir disoproxil fumarate (300 mg) under the brand name Delstrigo [2018]. Antiviral resistance can occur and resistance patterns are similar to, but somewhat less frequent than, those due to other nonnucleoside reverse transcriptase inhibitors. Common side effects include fatigue, dizziness, headache, diarrhea, nausea, disturbed sleep, abnormal dreams and skin rashes. Uncommon, but potentially severe adverse events include hypersensitivity reactions and immune reconstitution syndrome.
Hepatotoxicity
Serum aminotransferase elevations were reported in 13% of patients on doravirine therapy, but elevations above 5 times the upper limit of normal were uncommon, occurring in 1% or less of patients. The rate of serum aminotransferase elevations during doravirine therapy was higher in patients who were coinfected with hepatitis B or C, but the abnormalities were rarely severe. Doravirine has been in clinical use for a short time only, but unlike many other nonnucleoside reverse transcriptase inhibitors, doravirine has yet to be linked to reported instances of clinically apparent liver injury.
Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury).
Mechanism of Injury
Clinically apparent hepatotoxicity from doravirine has not been reported, but liver injury from other nonnucleoside reverse transcriptase inhibitors appears to be due to hypersensitivity. Doravirine is extensively metabolized in the liver via the cytochrome P450 system (predominantly CYP 3A4) and is susceptible to drug-drug interactions with modulators of CYP 3A4. Its use with strong CYP 3A4 inducers should be avoided because of potential decreases in doravirine plasma concentrations.
Outcome and Management
Doravirine therapy has been linked to mild and self-limited serum aminotransferase elevations which rarely require dose modification or discontinuation. Doravirine has not been linked to cases of acute hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between doravirine and other nonnucleoside reverse transcriptase inhibitors, although there may be cross reactivity in occurrence of rash.
Drug Class: Antiviral Agents, Antiretroviral Agents
Other Drugs in the Subclass, Nonnucleoside Reverse Transcriptase Inhibitors: Delavirdine, Efavirenz, Etravirine, Nevirapine, Rilpivirine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Doravirine – Pifeltro®
Doravirine, Lamivudine, Tenofovir disoproxil fumarate – Delstrigo®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Doravirine | 1338225-97-0 | C17-H11-Cl-F3-N5-O3 |
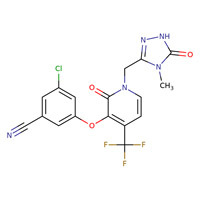 |
ANNOTATED BIBLIOGRAPHY
References updated: 12 April 2019
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents, but does not include a discussion of doravirine).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1137-48.(Textbook of pharmacology and therapeutics).
- http://aidsinfo
.nih.gov/guidelines/ (Regularly updated clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy). - Torti C, Lapadula G, Casari S, Puoti M, Nelson M, Quiros-Roldan E, Bella D, et al.; EPOKA-MASTER Study Group. Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients: results from the Italian EPOKA-MASTER Cohort. BMC Infect Dis 2005; 5: 58. [PMC free article: PMC1188059] [PubMed: 16018804](Among 1038 HIV-HCV coinfected patients starting antiretroviral therapy, the risk of ALT elevations above 5 times ULN was 17.1% yearly in treatment-naïve and 8.2% in treatment-experienced patients, risk factors being baseline ALT levels and use of nonnucleoside reverse transcriptase inhibitors).
- Labarga P, Soriano V, Vispo ME, Pinilla J, Martin-Carbonero L, Castellares C, Casado R, et al. Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J Infect Dis 2007; 196: 670-6. [PubMed: 17674307](Among 133 patients with HIV-HCV coinfection who were treated with interferon or peginterferon, 33% had a sustained response and subsequent yearly rate of hepatic events was higher among nonresponders [12.9%] than responders [3.1%]; also more common with dideoxynucleosides).
- Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother 2007; 59: 342-6. [PubMed: 17255142](Review of liver toxicity of nevirapine and efavirenz, ALT elevations >5 times ULN reported in 1-8% of efavirenz compared to 4-16% of nevirapine recipients; no mention of delavirdine or rilpivirine).
- Soriano V, Puoti M, Garcia-Gascó Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS 2008; 22: 1-13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management, stopping therapy if symptoms arise, with overt jaundice [direct bilirubin], evidence of mitochondrial toxicity, ALT >10 times ULN, ALT at lower levels if newly marketed agent; important to rule out other causes; problematic agents include didanosine, stavudine and zidovudine, nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiretroviral agent, but none to rilpivirine or doravirine).
- Colombier MA, Molina JM. Doravirine: a review. Curr Opin HIV AIDS 2018; 13: 308-14. [PubMed: 29794817](Review of the structure, mechanism of action, pharmacology, drug-drug interactions, antiviral resistance patterns, clinical efficacy and safety of doravirine; no specific discussion of ALT elevations or hepatotoxicity).
- Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, Lai MT, et al.; DRIVE-FORWARD Study Group. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV 2018; 5: e211-e220. [PubMed: 29592840](Among 769 HIV infected patients treated with doravirine vs darunavir/R combined with 2 antiretroviral nucleoside analogues, rates of HIV RNA suppression were similar [84% vs 80%], and common side effects included diarrhea, nausea and headache, ALT elevations above 5 times ULN occurring in 1% vs 2% and not requiring discontinuation in any doravirine-treated subject).
- Orkin C, Squires KE, Molina JM, Sax PE, Wong WW, Sussmann O, Kaplan R, et al.; DRIVE-AHEAD Study Group. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis 2019; 68: 535-44. [PMC free article: PMC6355823] [PubMed: 30184165](Among 728 HIV infected patients treated with doravirine vs efavirenz [both combined with 2 antiretroviral nucleoside analogues], rates of viral suppression were similar [84% vs 81%] while adverse events were less with doravirine including drug-related adverse events [31% vs 63%], serious adverse events [4% vs 6%], rash [5% vs 12%] and ALT elevations above 5 times ULN [0.6% vs 1.4%]).
- Deeks ED. Doravirine: first global approval. Drugs 2018; 78: 1643-50. [PubMed: 30341683](Review of the structure, pharmacology, clinical efficacy, resistance patterns, and safety of doravirine shortly after its approval for use in the US; does not mention ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Rilpivirine.[LiverTox: Clinical and Researc...]Review Rilpivirine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Delavirdine.[LiverTox: Clinical and Researc...]Review Delavirdine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Evaluation of the Pharmacokinetics of Metformin Following Coadministration With Doravirine in Healthy Volunteers.[Clin Pharmacol Drug Dev. 2020]Evaluation of the Pharmacokinetics of Metformin Following Coadministration With Doravirine in Healthy Volunteers.Sanchez RI, Yee KL, Fan L, Cislak D, Martell M, Jordan HR, Iwamoto M, Khalilieh S. Clin Pharmacol Drug Dev. 2020 Jan; 9(1):107-114. Epub 2019 Apr 11.
- Impact of HIV-1 Resistance-Associated Mutations on Susceptibility to Doravirine: Analysis of Real-World Clinical Isolates.[Antimicrob Agents Chemother. 2...]Impact of HIV-1 Resistance-Associated Mutations on Susceptibility to Doravirine: Analysis of Real-World Clinical Isolates.Asante-Appiah E, Lai J, Wan H, Yang D, Martin EA, Sklar P, Hazuda D, Petropoulos CJ, Walworth C, Grobler JA. Antimicrob Agents Chemother. 2021 Nov 17; 65(12):e0121621. Epub 2021 Sep 27.
- Review Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection.[Drugs Context. 2020]Review Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection.Pham HT, Xiao MA, Principe MA, Wong A, Mesplède T. Drugs Context. 2020; 9. Epub 2020 Mar 3.
- Doravirine - LiverToxDoravirine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...