NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dicloxacillin is an oral, second generation penicillin antibiotic that is used to treat bacterial infections caused by penicillinase-resistant staphylococci. Dicloxacillin has been linked to rare instances of clinically apparent, idiosyncratic liver injury.
Background
Dicloxacillin (dye klox' a sil' in) is a second generation penicillin that is resistant to inactivation by penicillinases and is used to treat infections caused by penicillinase-producing bacteria. Like other penicillins, dicloxacillin is a beta lactam antibiotic that is believed to act by binding to the bacterial enzyme that is responsible for synthesizing peptidoglycans which are necessary for the integrity of the bacterial cell wall. Dicloxacillin was approved for use in the United States in 1968 and is still widely used to treat mild-to-moderate staphylococcal infections. To reduce development of drug resistant bacteria, dicloxacillin is recommended to treat or prevent only those infections that are proven or suspected to be caused by penicillinase-producing susceptible bacteria. Dicloxacillin is available in multiple generic forms as 250 and 500 mg capsules and as a suspension for pediatric use. The typical dose is 125 to 500 mg every 6 hours. Common side effects of dicloxacillin include nausea, diarrhea, stomatitis, skin rash and allergic reactions. Rare but potentially severe adverse events include anaphylaxis, Clostridium difficile diarrhea and neutropenia.
Hepatotoxicity
Dicloxacillin therapy has not been associated with serum enzyme elevations during treatment, but has been linked to rare instances of clinically apparent, cholestatic hepatitis. The typical time to onset is 1 to 6 weeks and the pattern of serum enzyme elevations is usually cholestatic, although cases with a mixed pattern have also been described (Case 1). The injury usually presents with jaundice and pruritus. Fever, rash and eosinophilia can occur, but are not prominent and autoantibodies are rarely detected. A similar pattern of injury occurs more frequently with flucloxacillin (also called floxacillin) and cloxacillin, two oral isoxazolyl penicillins similar in structure and activity to dicloxacillin, but never approved for use or available in the United States. Similar cholestatic hepatitis arising 1 to 6 weeks after starting therapy occurs with other penicillins.
Likelihood score: B (highly likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the idiosyncratic, cholestatic hepatitis following dicloxacillin therapy is not known. Allergic manifestations (rash, fever and eosinophilia) are not common and the liver injury is usually not accompanied by signs or symptoms of penicillin hypersensitivity. However, the rapid recurrence of injury with reexposure suggests a hypersensitivity mechanism, perhaps in response to the beta lactam ring. Injury occurs more frequently in older patients. Too few cases of dicloxacillin hepatotoxicity have been reported to comment on possible HLA associations, such as the link to HLA-B*57:01 which has been made to flucloxacillin.
Outcome and Management
In the few cases that have been described, cholestasis has been prolonged, but all patients recovered clinically within 6 to 12 weeks, but some had evidence of residual injury. Reexposure appears to be associated with recurrence of injury, often with a shortened latency period. The idiosyncratic liver injury from dicloxacillin has not been linked to acute liver failure or the vanishing bile duct syndrome (although these have been described with similar cases due to flucloxacillin). Prednisone has been used to treat the cholestatic liver injury, but its effects are unclear while its side effects can be serious. Patients should be told to avoid reexposure to the penicillinase-resistant penicillins, including nafcillin and oxacillin.
Drug Class: Penicillin (Penicillinase-Resistant)
CASE REPORT
Case 1. Cholestatic hepatitis caused by dicloxacillin.(1)
A 56 year old man received a 5 day course of oral dicloxacillin (250 mg four times a day) and 2 weeks later developed gastrointestinal discomfort, followed by fever and then jaundice and itching. He had a history of hepatitis 22 years ago, but no other significant medical history and had no current risk factors for viral hepatitis. He took no other medications, had no allergies and drank little alcohol. On admission, his serum bilirubin was 3.8; that later rose (Table). Eosinophil counts were normal. Tests for acute hepatitis A and B were negative and abdominal ultrasound was normal. After several weeks, he began to improve and laboratory abnormalities returned to normal 14 weeks after he had started the course of antibiotics.
Key Points
| Medication: | Dicloxacillin |
|---|---|
| Pattern: | Mixed (R=2.6) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | Two to three weeks |
| Recovery: | Complete in 3 months |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | AST* (U/L) | AST* (U/L) | Bilirubin* (mg/dL) | Comments |
|---|---|---|---|---|---|
| 0 | Dicloxacillin given for 5 days | ||||
| 3 weeks | 2 weeks | 225 | 218 | 3.8 | Eosinophils=487 |
| 4 weeks | 3 weeks | 325 | 230 | 8.0 | |
| 5 weeks | 4 weeks | 50 | 150 | 10.3 | |
| 7 weeks | 6 weeks | 45 | 165 | 11.8 | |
| 8 weeks | 7weeks | 60 | - | 9.8 | |
| 9 weeks | 8 weeks | 110 | - | 5.0 | |
| 10weeks | 9 weeks | 80 | 170 | 3.2 | |
| 12 weeks | 11 weeks | 70 | 135 | 1.8 | |
| 13 weeks | 12 weeks | 65 | 120 | 1.1 | |
| 14 weeks | 13 weeks | 38 | 60 | 0.5 | |
| Normal Values | <40 | <100 | <1.2 | ||
- *
Estimates made from Figure 1.
Comment
The appearance of jaundice and itching 3 weeks after starting a 5 day course of dicloxacillin with a mixed pattern of serum enzyme elevations (later becoming more cholestatic) is fully compatible with dicloxacillin induced liver injury. Recovery was somewhat slow, but was complete by 3 months. Idiosyncratic drug induced liver injury from dicloxacillin is rare and only isolated case reports have been published. More common and with a similar pattern of injury are cases of flucloxacillin (which was approved and used in Australia and Europe, but not in the United States). Flucloxacillin hepatotoxicity is characterized by a latency of 2 to 6 weeks (often arising 1 to 3 weeks after stopping therapy), with a cholestatic or mixed enzyme pattern and recovery in 1 to 3 months. Fatalities and vanishing bile duct syndrome have been reported with flucloxacillin, but not with dicloxacillin despite the similarity of the injury caused, probably because dicloxacillin is just much less likely to lead to hepatic injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dicloxacillin – Generic
DRUG CLASS
Penicillin (Penicillinase-Resistant)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dicloxacillin | 3116-76-5 | C19-H17-Cl2-N3-O5-S |
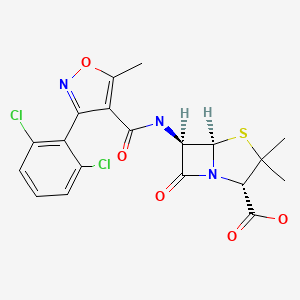
|
CITED REFERENCE
- 1.
- Kleinman MS, Presberg JE. Cholestatic hepatitis after dicloxacillin-sodium therapy. J Clin Gastroenterol. 1986;8:77–8. [PubMed: 3701014]
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Synthetic penicillins. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 596-8.(Expert review of penicillins and liver injury published in 1999).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that liver injury from the penicillins is very rare, and is usually cholestatic for the oxypenicillins such as dicloxacillin and cloxacillin).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Kleinman MS, Presberg JE. Cholestatic hepatitis after dicloxacillin-sodium therapy. J Clin Gastroenterol. 1986;8:77–8. [PubMed: 3701014](56 year old man developed jaundice and pruritus 2-3 weeks after a 5 day course of dicloxacillin [bilirubin rising to 13 mg/dL, AST 225 U/L, Alk P 318 U/L], without rash, eosinophilia or fever, requiring 10 weeks to resolve).
- Siegmund JB, Tarshis AM. Prolonged jaundice after dicloxacillin therapy. Am J Gastroenterol. 1993;88:1299–300. [PubMed: 8338117](36 year old woman developed fatigue and jaundice 3 weeks after starting dicloxacillin [bilirubin 18.2 mg/dL, ALT 100 U/L, Alk P 312 U/L], with slow recovery even with prednisone; 3 years later, enzymes were still mildly elevated [ALT 44 U/L, Alk P 188 U/L]).
- Saab S, Venkataramani A, Yao F. Possible granulomatous hepatitis after dicloxacillin therapy. J Clin Gastroenterol. 1996;22:163–4. [PubMed: 8742666](74 year old developed rash 5 days after starting oral dicloxacillin [bilirubin 0.5 mg/dL, ALT 172 U/L, Alk P 183 U/L], biopsy showing a single granuloma and mild nonspecific changes, ultimately resolving within 4 months of stopping).
- Ibáñez L, Pérez E, Vidal X, Laporte JR. Grup d'Estudi Multicènteric d'Hepatotoxicitat Aguda de Barcelona (GEMHAB). Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol. 2002;37:592–600. [PubMed: 12399224](Survey of 107 cases of acute serious liver disease, not due to viruses, found no instances of drug induced liver injury due to penicillinase-resistant penicillins).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Analysis of all fatal adverse drug event reports of liver injury in Sweden between 1966 and 2002, found 103 cases; most common causes were halothane [n=16], acetaminophen [14], flucloxacillin [9], and TMP-SMZ [6]).
- Hussaini SH, O'Brien CS, Despott EJ, Dalton HR. Antibiotic therapy: a major cause of drug-induced jaundice in southwest England. Eur J Gastroenterol Hepatol. 2007;19:15–20. [PubMed: 17206072](Review of causes of non-obstructive jaundice in 347 patients presenting between 1998 and 2004 at a single UK center, 28 were thought to be drug induced liver injury [8.1%] and antibiotics were the most common cause, 32% amoxicillin/clavulanate, 25% flucloxacillin, 18% other).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, antimicrobials accounted for 45% of cases with 23 single agent cases due to amoxicillin/clavulanate, 13 nitrofurantoin, 10 fluoroquinolones, 9 macrolides, 9 sulfonamides, 5 cephalosporins, 3 oxacillin, 2 doxycycline, 2 amoxicillin, and one each for gentamicin, imipenem, and clindamycin, but none from dicloxacillin or nafcillin).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; leading causes were antituberculosis agents [58%], anticonvulsants [11%] and NSAIDs [2%]; specific antibiotic agents included sulfamethoxazole/trimethoprim [2%] and amoxicillin-clavulanate [1%]).
- Ferrajolo C, Capuano A, Verhamme KMC, Schuemie M, Rossi F, Stricker BH, Sturkenboom CJM. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse drug reports in children in a worldwide pharmacovigilance database, 6595 [1%] were for hepatic injury and antibacterials accounted for 11%, those with the highest adjusted odds ratios being aztreonam, erythromycin, ceftriaxone and minocycline; no mention of penicillins).
- Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, Daly MJ, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature Genetics. 2009;41:816–9. [PubMed: 19483685](Genome-wide association study [GWAS] in 51 cases of flucloxacillin liver injury and 285 controls found an association with a single nucleotide polymorphism linked to HLA-B*5701 [84% in cases vs 5% in controls], the same genotype associated with abacavir hypersensitivity; ~0.2% of persons with this HLA type receiving flucloxacillin develop cholestatic hepatitis, making it likely that other genes or factors are important as well).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 66 due to antimicrobial agents, but none were attributed to a penicillinase-resistant penicillin).
- Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11:241–6. [PubMed: 20300088](Review of recent advances in GWAS studies of pharmacokinetics and adverse drug reactions and association of flucloxacillin injury with HLA-B*5701).
- Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, Alfirevic A, et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–39. [PubMed: 22987284](T cells from persons with HLA-B*57:01 were activated when exposed to dendritic cells presenting flucloxacillin bound to albumin, and were similarly activated by oxacillin, cloxacillin and dicloxacillin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin/clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, but none to penicillinase resistant penicillins such as oxacillin or dicloxacillin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 cases [36%] were attributed to antibiotics 3 of which were due to oxacillin, all being self-limited episodes of aminotransferase elevations without jaundice; no instances of dicloxacillin or nafcillin associated liver injury).
- Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome-wide association study done on 862 patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in patients with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–1716.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome-wide association studies on 2048 patients with drug-induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [DICLOXACILLIN, A NEW, ACID-STABILE AND PENICILLINASE-RESISTANT ORAL PENICILLIN].[Arzneimittelforschung. 1965][DICLOXACILLIN, A NEW, ACID-STABILE AND PENICILLINASE-RESISTANT ORAL PENICILLIN].NAUMANN P, KEMPF B. Arzneimittelforschung. 1965 Feb; 15:139-45.
- Review Oxacillin.[LiverTox: Clinical and Researc...]Review Oxacillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- IL-4 mediates dicloxacillin-induced liver injury in mice.[Toxicol Lett. 2011]IL-4 mediates dicloxacillin-induced liver injury in mice.Higuchi S, Kobayashi M, Yoshikawa Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Toxicol Lett. 2011 Feb 5; 200(3):139-45. Epub 2010 Nov 19.
- Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin.[Br Med J. 1970]Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin.Sutherland R, Croydon EA, Rolinson GN. Br Med J. 1970 Nov 21; 4(5733):455-60.
- Review Nafcillin.[LiverTox: Clinical and Researc...]Review Nafcillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Dicloxacillin - LiverToxDicloxacillin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...