NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cycloserine is a broad spectrum antibiotic used as a second line agent for treatment of drug resistant tuberculosis, always in combination with other antituberculosis agents. Cycloserine is appears to have little or no hepatotoxic potential, but it is usually used in combination with agents that are known to be hepatotoxic, and its role in the reported cases of liver injury with combination therapy cannot always be excluded.
Background
Cycloserine (sye" kloe ser' een) is an antibiotic that is currently used largely in the therapy of tuberculosis caused by multidrug resistant mycobacteria. Cycloserine is a d-alanine analogue of isoxazolidone that was isolated initially from Streptococcus orchidaceus and has moderate activity in vitro against mycobacterial species, probably acting by inhibition of mycobacterial use of amino acids and inhibition of cell wall synthesis. Importantly, there is no cross resistance between cycloserine and most other medications for tuberculosis. Cycloserine was approved for use in the United States in 1964, but its use for most indications has been replaced by more modern antituberculosis agents except in instances of multidrug resistance or of intolerance to the more potent agents such as isoniazid, rifampin and pyrazinamide. Cycloserine is available in tablets of 250 mg in generic forms and under the brand name Seromycin; the typical adult dose is 250 to 500 mg twice daily (~10-15 mg/kg/day). Common side effects are drowsiness, headache, fatigue, rash and fever; rarely, cycloserine causes more serious neurological side effects such as acute psychosis, seizures and coma. Regularly updated information on guidelines for therapy of tuberculosis is available at the Centers for Disease Control and Prevention website:
http://www.cdc.gov/tb/publications/guidelines/Treatment.htm.
Hepatotoxicity
Cycloserine is reported to be associated with a low rate of serum aminotransferase elevations that are usually transient and asymptomatic and do not require dose modification. Cycloserine is usually used in combination with agents that are more clearly linked to liver test abnormalities, and it generally plays little or no role in these abnormalities. Cycloserine has not been definitely linked to instances of clinically apparent liver injury, but is often used with agents that are known hepatotoxins and its possible contribution cannot always be excluded.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
Cycloserine undergoes minimal hepatic metabolism, perhaps accounting for the absence of significant hepatotoxicity. Allergic reactions have been reported with cycloserine and, if severe, these may be accompanied by mild serum enzyme elevations.
Outcome and Management
Cycloserine blood levels are typically monitored during therapy and the product label recommends monitoring of blood counts, renal function and routine liver tests as well.
[First line medications used in the therapy of tuberculosis in the US include ethambutol, isoniazid, pyrazinamide, rifabutin, rifampin, and rifapentine. Second line medications include streptomycin, capreomycin, cycloserine, ethionamide, fluoroquinolones such as levofloxacin and moxifloxacin, aminoglycosides such as amikacin, and para-aminosalicylic acid (PAS).]
Drug Class: Antituberculosis Agents
Other Drugs in the Class: Bedaquiline, Capreomycin, Ethambutol, Ethionamide, Isoniazid, Pyrazinamide, Rifabutin, Rifampin, Rifapentine, Streptomycin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cycloserine – Generic, Seromycin®
DRUG CLASS
Antituberculosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
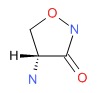
| Cycloserine | 68-41-7 | C3-H6-N2-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 November 2017
- Zimmerman HJ. Antituberculosis agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 611-21.(Extensive review of hepatotoxicity of antituberculosis medications published in 1999 states that: “Several drugs employed as second-line or supplementary agents for the treatment of tuberculosis [cycloserine, capreomycin] apparently produce little or no hepatic injury”).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504.(Review of hepatotoxicity of antituberculosis drugs).
- Gumbo T. Chemotherapy of tuberculosis, mycobacterium avium complex disease and leprosy. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1549-70.(Textbook of pharmacology and therapeutics).
- Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis 1996; 77: 37-42. [PubMed: 8733412](Among 1317 patients treated for active tuberculosis, hepatitis was attributed to rifampin in 1.4%, pyrazinamide in 1.2% and isoniazid in 0.3%, but none to cycloserine, ethambutol or streptomycin).
- American Thoracic Society; Centers for Disease Control and Prevention (CDC); Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep 2003; 52 (RR-11): 1-77. [PubMed: 12836625](Recommendations for therapy of tuberculosis including details of drug regimens, side effects, monitoring and optimal approaches to follow up; cycloserine is considered a second line agent, used largely for cases of drug resistant tuberculosis or in situations in which the first line agents are contraindicated or poorly tolerated).
- Di Perri, Bonora S. Which agents should we use for the treatment of multidrug-resistant Mycobacterium tuberculosis? J Antimicrob Chemother 2004; 54: 593-602. [PubMed: 15282233](Multidrug resistance is defined as an organism resistant to at least isoniazid and rifampin; authors rank second-line agents as: (1) levofloxacin, aminoglycosides, and capreomycin, (2) ethionamide, ofloxacin and ciprofloxacin, (3) PAS, (4) cycloserine, (5) amoxicillin/clavulate or ampicillin/sulbactam, (6) clarithromycin, linezolid and clofazimine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, isoniazid was implicated in 15 cases [5% of cases overall, ranking 3rd, 13 as a single agent and 2 in combination with other agents], but no case was attributed to cycloserine).
- Abubakar I, Moore J, Drobniewski F, Kruijshaar M, Brown T, Yates M, Anderson C, et al. Extensively drug-resistant tuberculosis in the UK: 1995 to 2007. Thorax 2009; 64: 512-5. [PubMed: 19318348](Among 678 extensively drug resistant isolates of tuberculosis reported in the UK between 2005 and 2008, 5.1% were resistant to cycloserine).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 25 to antituberculosis agents, including 15 to isoniazid alone [ranking first], 6 to isoniazid combined with other agents, 3 to rifampin and pyrazinamide and 1 to dapsone, none to cycloserine).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396-404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen between 1997 and 2008 at a large hospital in Bangalore, India, 181 [58%] were attributed to antituberculosis agents which accounted for 39 of 54 [72%] fatal cases, cycloserine was not specifically mentioned).
- Arbex MA, Varella Mde C, Siqueira HR, Mello FA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 2: second line drugs. J Bras Pneumol 2010; 36: 641-56. [PubMed: 21085831](Analysis of adverse effects of second line drugs for tuberculosis; cycloserine has neurological adverse effects which can be severe, but no mention is made of hepatotoxicity).
- Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 2010; 10: 621-9. [PubMed: 20797644](Review of drug-resistance in tuberculosis mentions that cycloserine is a cornerstone of treatment for multidrug resistant strains, major drawbacks being its neurological side effects and its short shelf-life; no mention of hepatotoxicity).
- Palmero D, Castagnino J, Musella RM, Mosca C, González Montaner P, de Casado GC. Difficult clinical management of anti-tuberculosis DRESS syndrome. Int J Tuberc Lung Dis 2013; 17: 76-8. [PubMed: 23114284](Analysis of 11 patients with DRESS syndrome attributed to antituberculosis medications including isoniazid, rifampin, ethambutol, pyrazinamide and others usually in combination, but none were receiving cycloserine).
- Kaswala DH. Drug rash with eosinophilia and systemic symptoms syndrome due to anti-TB medication. J Family Med Prim Care 2013; 2: 83-5. PubMed Citation. [PMC free article: PMC3894017] [PubMed: 24479051](68 year old man with pulmonary tuberculosis developed skin rash, eosinophlia and liver test abnormalities 8 weeks after starting isoniazid, pyrazinamide, rifampin and ethambutol, which resolved within 4 weeks of stopping and with corticosteroid therapy, and did not recur when treated with cycloserine and moxifloxacin).
- Bhushan B, Chander R, Kajal NC, Ranga V, Gupta A, Bharti H. Profile of adverse drug reactions in drug resistant tuberculosis from Punjab. Indian J Tuberc 2014; 61: 318-24. PubMed Citation. [PubMed: 25675695](Among 207 patients with drug resistant tuberculosis with an adverse drug reaction while on second line regimen, there were 24 instances of hepatitis which were severe and required discontinuation in 5, 3 of which were attributed to pyrazinamide, recurring with restarting; no mention of cycloserine).
- Tandon VR, Rani N, Roshi, Gupta R, Arora M, Khajuria V, Mahajan V. Cycloserine induced psychosis with hepatic dysfunction. Indian J Pharmacol 2015; 47: 230-1. PubMed Citation. [PMC free article: PMC4386140] [PubMed: 25878391](45 year old man with multidrug resistant pulmonary tuberculosis developed acute psychosis 18 months after starting a second line regimen that included cycloserine, the abnormal behavior resolving with stopping and reappearing with restarting cycloserine at which time abnormal liver test results were identified [bilirubin 5.4 mg/dL, ALT 202 U/L, Alk P not given], subsequent course not described).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 53 cases [6%] were attributed to antituberculosis medications, but none to cycloserine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Molecular basis underlying Mycobacterium tuberculosis D-cycloserine resistance. Is there a role for ubiquinone and menaquinone metabolic pathways?[Expert Opin Ther Targets. 2014]Review Molecular basis underlying Mycobacterium tuberculosis D-cycloserine resistance. Is there a role for ubiquinone and menaquinone metabolic pathways?Hong W, Chen L, Xie J. Expert Opin Ther Targets. 2014 Jun; 18(6):691-701. Epub 2014 Apr 29.
- Review Streptomycin.[LiverTox: Clinical and Researc...]Review Streptomycin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Antituberculosis Agents.[LiverTox: Clinical and Researc...]Review Antituberculosis Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Efficacy and safety of kanamycin, ethionamide, PAS and cycloserine in multidrug-resistant pulmonary tuberculosis patients.[Indian J Chest Dis Allied Sci....]Efficacy and safety of kanamycin, ethionamide, PAS and cycloserine in multidrug-resistant pulmonary tuberculosis patients.Prasad R, Verma SK, Sahai S, Kumar S, Jain A. Indian J Chest Dis Allied Sci. 2006 Jul-Sep; 48(3):183-6.
- Pharmacokinetic Modeling, Simulation, and Development of a Limited Sampling Strategy of Cycloserine in Patients with Multidrug-/Extensively Drug-Resistant Tuberculosis.[Clin Pharmacokinet. 2020]Pharmacokinetic Modeling, Simulation, and Development of a Limited Sampling Strategy of Cycloserine in Patients with Multidrug-/Extensively Drug-Resistant Tuberculosis.van der Galiën R, Boveneind-Vrubleuskaya NV, Peloquin C, Skrahina A, Touw DJ, Alffenaar JC. Clin Pharmacokinet. 2020 Jul; 59(7):899-910.
- Cycloserine - LiverToxCycloserine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...