NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Capreomycin is an injectable broad spectrum antibiotic used in the therapy of drug resistant tuberculosis as a second line agent, always in combination with other antituberculosis drugs. Capreomycin has not been linked to cases of clinically apparent liver disease.
Background
Capreomycin (kap" ree oh mye' sin) is a cyclic peptide antibiotic that was initially isolated from the bacterium, Streptomyces capreolus. Capreomycin is believed to act by binding to bacterial ribosomes and inhibiting protein synthesis. Capreomycin is similar to viomycin and is considered bacterostatic rather than bacterocidal. While structurally different, capreomycin has similar antibacterial activity and side effects to the aminoglycosides (streptomycin, kanamycin, amikacin). Capreomycin is currently used only as a secondary agent in the treatment of active tuberculosis, always in combination with other antituberculosis agents such as isoniazid, ethambutol, pyrazinamide and/or rifampin (but not streptomycin or the aminoglycosides) and usually for multidrug resistant mycobacterial infections. Capreomycin was approved for use in the United States in 1973, but is currently rarely used, streptomycin or a modern aminoglycoside being used in its place. Capreomycin is available as lyophilized powder for suspension available in vials of 1 gram for deep intramuscular or intravenous injection under the trade name of Capastat. The typical dose is 15 mg/kg/day (maximum of 1 gram per day) intramuscularly for 2 to 4 months and it is always given in combination with other antituberculosis agents (which are typically continued for 12 to 24 months). The dose must be modified based upon renal function. Side effects resemble those of the aminoglycosides and include tinnitus, hearing loss, proteinuria and renal dysfunction, and local injection reactions.
Guidelines and detailed discussion of therapy of tuberculosis is available at the Centers for Disease Control and Prevention website: https://www.cdc.gov/tb/publications/guidelines/Treatment.htm
Hepatotoxicity
Intramuscular therapy with capreomycin has not been definitely linked to liver injury, either in the form of asymptomatic elevations in serum enzymes or of clinically apparent liver injury. However, the agent is rarely used and is always used in combination with other antituberculosis medications, many of which are well known to cause liver injury. Nevertheless, in the few studies done, capreomycin has not been associated with an increase in the rate of serum enzyme elevations or in episodes of acute liver injury above or beyond what was reported in the comparator arm.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Capreomycin is excreted largely unchanged in the urine. Its lack of hepatic metabolism and known oto- and nephrotoxicity (which are dose-limiting) may account for its lack of hepatotoxicity. The majority of toxicities of capreomycin resemble those of streptomycin and the aminoglycosides and include oto- and nephrotoxicity, electrolyte disturbances, hypersensitivity reactions, and local injection reactions.
[First line medications used in the therapy of tuberculosis in the US include ethambutol, isoniazid, pyrazinamide, rifabutin, rifampin, and rifapentine. Second line medications include streptomycin, capreomycin, cycloserine, ethionamide, fluoroquinolones such as levofloxacin and moxifloxacin, aminoglycosides such as amikacin, and para-aminosalicylic acid (PAS).]
Drug Class: Antituberculosis Agents
Other Drugs in the Class: Bedaquiline, Cycloserine, Ethambutol, Ethionamide, Isoniazid, Pyrazinamide, Rifabutin, Rifampin, Rifapentine, Streptomycin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Capreomycin – Capastat®
DRUG CLASS
Antituberculosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Capreomycin | 11003-38-6 | Unspecified |
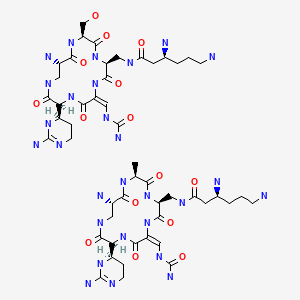
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 September 2021
- Zimmerman HJ. Antituberculosis agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 611-21.(Extensive review of hepatotoxicity of antituberculosis medications published in 1999: “Several drugs employed as second line or supplementary agents for the treatment of tuberculosis [cycloserine, capreomycin] apparently produce little or no hepatic injury”).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504.(Review of hepatotoxicity of antituberculosis drugs).
- Gumbo T. Chemotherapy of tuberculosis, mycobacterium avium complex disease and leprosy. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1067-86.(Textbook of pharmacology and therapeutics).
- Hellström PE, Repo UK. Capreomycin, ethambutol and rifampicin in apparently incurable pulmonary tuberculosis. Scand J Respir Dis Suppl. 1969;69:69–74. [PubMed: 4906377](Retrospective analysis of 35 patients with severe, chronic or relapsing tuberculosis who were treated with capreomycin, ethambutol and rifampin; “liver damage” occurred in 49% but was reversible in all; details not given, usually attributed to rifampin).
- Wäre M, Heinivaara O, Elo R, Tala E. Clinical experience of the treatment of drug-resistant pulmonary tuberculosis with rifampicin combined with ethambutol and capreomycin. Scand J Respir Dis Suppl. 1969;69:59–63. [PubMed: 4906375](Among 58 patients with multidrug resistant tuberculosis treated with capreomycin combined with ethambutol and rifampin, 2 developed AST elevations, both attributed to rifampin, but stopping both drugs led to improvements in both patients).
- Repo UK, Hellström PE. Capreomycin and ethambutol in pulmonary tuberculosis. A preliminary report. Scand J Respir Dis Suppl. 1970;72:72–5. [PubMed: 5273730](Retrospective analysis of 29 patients with severe tuberculosis treated with capreomycin and ethambutol with a third agent; “liver damage” occurred in 10%, but was reversible in all and consisted of mild elevations in ALT, AST and BSP retention).
- Aquinas M, Citron KM. Rifampicin, ethambutol and capreomycin in pulmonary tuberculosis, previously treated with both first and second line drugs: the results of 2 years chemotherapy. Tubercle. 1972;53:153–65. [PubMed: 4116993](Among 40 patients with active tuberculosis treated with capreomycin for 6 months and rifampin and ethambutol for 2 years, ALT elevations occurred in 2 patients, one with jaundice resolving after stopping rifampin and one with asymptomatic elevations resolving spontaneously without dose modification).
- Anonymous Evaluation of a new antituberculous agent. Capreomycin sulfate (capastat sulfate). JAMA. 1973;223:179–80. [PubMed: 4120232](Short summary of capreomycin including pharmacology, indications, and safety).
- Byrd RB, Kaplan PD, Gracey DR. Treatment of pulmonary tuberculosis. Chest. 1974;66:560–7. [PubMed: 4139002](Review of therapy of tuberculosis; capreomycin is discussed as one of four injectable agents [along with streptomycin, kanamycin, and viomycin], their major toxicities being auditory and vestibular).
- American Thoracic Society. Centers for Disease Control and Prevention (CDC). Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed: 12836625](Recommendations for therapy of tuberculosis including details of drug regimens, side effects, monitoring and optimal approaches to follow up; capreomycin is a second line agent that must be given by parenteral injection).
- Di Perri Bonora S. Which agents should we use for the treatment of multidrug-resistant Mycobacterium tuberculosis? J Antimicrob Chemother. 2004;54:593–602. [PubMed: 15282233](Multidrug resistance is defined as an organism resistant to at least isoniazid and rifampin; authors rank second line agents as: 1. levofloxacin, aminoglycosides, and capreomycin, 2. ethionamide, ofloxacin and ciprofloxacin, 3. PAS, 4. cycloserine, 5. amoxicillin/clavulanate or ampicillin/sulbactam, 6. clarithromycin, linezolid and clofazimine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, no case was attributed to capreomycin and only 1 case to gentamicin).
- Abubakar I, Moore J, Drobniewski F, Kruijshaar M, Brown T, Yates M, Anderson C, et al. Extensively drug-resistant tuberculosis in the UK: 1995 to 2007. Thorax. 2009;64:512–5. [PubMed: 19318348](Among 678 extensively drug resistant isolates of tuberculosis reported in the UK between 2005 and 2008, 3.4% were also resistant to capreomycin and 51% to streptomycin).
- Arbex MA, Varella Mde C, Siqueira HR, Mello FA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 2: second line drugs. J Bras Pneumol. 2010;36:641–56. [PubMed: 21085831](Analysis of adverse effects of second line drugs for tuberculosis; while structurally different, capreomycin has similar antibacterial activity and adverse effects to the aminoglycosides, common side effects being nephro- and ototoxicity, electrolyte disturbances, local reactions, urticaria and skin rash; no mention of hepatotoxicity).
- Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–9. [PubMed: 20797644](Recommends that treatment of multidrug resistant tuberculosis should always include an injectable drug, the first choice being capreomycin, others being the aminoglycosides streptomycin, kanamycin and amikacin).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 25 to antituberculosis agents, including 15 to isoniazid alone [ranking first], 6 to isoniazid combined with other agents, 3 to rifampin and pyrazinamide and 1 to dapsone, not to capreomycin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 53 [6%] implicated drugs for tuberculosis including 48 attributed to isoniazid, but none to capreomycin or the aminoglycosides).
- Garcia-Prats AJ, Schaaf HS, Hesseling AC. The safety and tolerability of the second line injectable antituberculosis drugs in children. Expert Opin Drug Saf. 2016;15:1491–1500. [PubMed: 27548570](Review of the second line injectable drugs for tuberculosis including capreomycin, discusses ototoxicity, nephrotoxicity, electrolyte disturbances, local injection site reactions, but not hepatotoxicity).
- Arnold A, Cooke GS, Kon OM, Dedicoat M, Lipman M, Loyse A, Chis Ster I, et al. Adverse effects and choice between the injectable agents amikacin and capreomycin in multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61:e02586–16. [PMC free article: PMC5571306] [PubMed: 28696239](Among 100 adults with multidrug resistant tuberculosis treated with injectable agents for a median of 6 months, ototoxicity arose in 5% of those on capreomycin vs 52% on amikacin and resulted in early discontinuation in 40% of subjects; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Adverse Effects and Choice between the Injectable Agents Amikacin and Capreomycin in Multidrug-Resistant Tuberculosis.[Antimicrob Agents Chemother. 2...]Adverse Effects and Choice between the Injectable Agents Amikacin and Capreomycin in Multidrug-Resistant Tuberculosis.Arnold A, Cooke GS, Kon OM, Dedicoat M, Lipman M, Loyse A, Chis Ster I, Harrison TS. Antimicrob Agents Chemother. 2017 Sep; 61(9). Epub 2017 Aug 24.
- Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis.[Int J Antimicrob Agents. 2017]Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis.Quenard F, Fournier PE, Drancourt M, Brouqui P. Int J Antimicrob Agents. 2017 Aug; 50(2):252-254. Epub 2017 Jun 5.
- Review The safety and tolerability of the second-line injectable antituberculosis drugs in children.[Expert Opin Drug Saf. 2016]Review The safety and tolerability of the second-line injectable antituberculosis drugs in children.Garcia-Prats AJ, Schaaf HS, Hesseling AC. Expert Opin Drug Saf. 2016 Nov; 15(11):1491-1500. Epub 2016 Aug 22.
- Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: a new approach to therapy of drug-resistant tuberculosis.[Antimicrob Agents Chemother. 2...]Phase I, single-dose, dose-escalating study of inhaled dry powder capreomycin: a new approach to therapy of drug-resistant tuberculosis.Dharmadhikari AS, Kabadi M, Gerety B, Hickey AJ, Fourie PB, Nardell E. Antimicrob Agents Chemother. 2013 Jun; 57(6):2613-9. Epub 2013 Mar 25.
- Review Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis.[Lancet Infect Dis. 2010]Review Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Lancet Infect Dis. 2010 Sep; 10(9):621-9.
- Capreomycin - LiverToxCapreomycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...