NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Carboplatin is an intravenously administered platinum coordination complex and alkylating agent which is used as a chemotherapeutic agent for the treatment of various cancers, mainly ovarian, head and neck and lung cancers. Carboplatin therapy is associated with a low rate of transient serum aminotransferase elevations and with rare instances of clinically apparent liver injury.
Background
Carboplatin (kar" boe pla' tin) is a cisplatin analog with a carboxy-cyclobutane moiety instead of the chloride atoms which makes it more stable and perhaps less toxic than cisplatin. Carboplatin and cisplatin act as alkylating agents causing cross linking between and within DNA strands, leading to inhibition of DNA, RNA and protein synthesis and the triggering of programmed cell death, mostly in rapidly dividing cells. Carboplatin was approved for use in cancer chemotherapy in the United States in 1989. It is currently indicated for advanced ovarian carcinoma, but is also used in other solid tumors including lung and head and neck cancer. Carboplatin is available in a powder or aqueous solution for injection in 50, 150 and 450 mg amounts generically and under the brand name Paraplatin. The platinum coordinating complexes have similar toxicities, including nausea and vomiting, diarrhea, bone marrow suppression, as well as neuro-, oto- and nephrotoxicity. They are also mutagenic, teratogenic and carcinogenic. Carboplatin is somewhat better tolerated than cisplatin.
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels are found in up to one-third of patients taking carboplatin. However, clinically apparent acute liver injury from carboplatin is extremely rare and the characteristics of such injury have not been well defined. In addition, carboplatin has been used in combination with other alkylating agents in high doses in conditioning regimens in preparation of hematopoietic cell transplantation which may be associated with instances of sinusoidal obstruction syndrome, which can be severe and lead to acute liver failure. Onset of sinusoidal obstruction syndrome is generally within 10 to 20 days of transplantation and presents with right upper quadrant pain, hepatic tenderness, weight gain, edema and ascites, followed by jaundice. The role of carboplatin in causing these cases of sinusoidal obstruction syndrome has not been well defined.
Carboplatin is usually given in combination with other antineoplastic agents and adverse events that occur with these combinations cannot always be attributed to carboplatin. In this regard, individual case reports of reactivation of hepatitis B, acute hepatic necrosis, sinusoidal obstruction syndrome and severe hyperammonemic coma (without liver injury) have been described after chemotherapeutic regimens that include carboplatin and other platinum coordination complexes such as cisplatin and oxaliplatin. In most of these instances, it is difficult to assign a specific role for carboplatin in causing the injury.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The cause of idiosyncratic hepatotoxicity from carboplatin is not known, but is possibly due to an intermediate in its metabolism. Sinusoidal obstruction syndrome is probably due to direct toxic effects of the myeloablative regimen on sinusoidal lining cells.
Outcome and Management
The severity of liver injury from carboplatin ranges from mild, reversible enzyme elevation to sinusoidal obstruction syndrome with acute liver failure and death. Liver injury from carboplatin is extremely rare. There is likely to be cross sensitivity to liver toxicities of the various platinum coordination complexes and rechallenge after clinically apparent liver injury should be avoided.
Drug Class: Antineoplastic Agents, Alkylating Agents
Other Drugs in the Subclass, Platinum Coordination Complexes: Cisplatin, Oxaliplatin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Carboplatin – Generic, Paraplatin®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Carboplatin | 41575-94-4 | C6-H12-N2-O4-Pt |
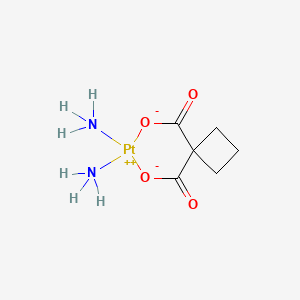
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 September 2020
Abbreviations: BMI, body mass index; CT, computerized tomography; NRH, nodular regenerative hyperplasia; SOS, sinusoidal obstruction syndrome; HVPG, hepatic venous pressure gradient; SAMe, S-adenosylmethionine.
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that cisplatin had been reported to cause dose related serum enzyme elevations and has been linked to steatosis and necrosis, whereas carboplatin has been linked to rare instances of cholestatic and hepatocellular injury).
- DeLeve LD. Liver sinusoidal endothelial cells and liver injury. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 139-43.(Review of liver injury to sinusoidal endothelial cells caused by medications mentions that oxaliplatin as capable of causing sinusoidal dilatation, peliosis hepatis, nodular regenerative hyperplasia and sinusoidal obstruction syndrome).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-201.(Textbook of pharmacology and therapeutics).
- Canetta R, Franks C, Smaldone L, Bragman K, Rozencweig M. Clinical status of carboplatin. Oncology (Williston Park). 1987;1:61–70. [PubMed: 3079484](Summary of clinical studies of carboplatin, a cisplatin derivative; carboplatin and cisplatin have similar efficacy against ovarian, cervical and small cell lung cancer, but carboplatin is better tolerated; ALT elevations in 16% of patients, bilirubin in 4%, no mention of clinically apparent hepatotoxicity).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. [PubMed: 3321587](Among 235 patients undergoing bone marrow transplantation between 1982 and 1985, SOS developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Canetta R, Bragman K, Smaldone L, Rozencweig M. Carboplatin: current status and future prospects. Cancer Treat Rev. 1988;15:17–32. [PubMed: 2841021](Summary of results of prelicensure trials of carboplatin; no mention of ALT elevations or hepatotoxicity).
- Hruban RH, Sternberg SS, Meyers P, Fleisher M, Menendez-Botet C, Boitnott JK. Fatal thrombocytopenia and liver failure associated with carboplatin therapy. Cancer Invest. 1991;9:263–8. [PubMed: 1913229](18 year old man with acute lymphocyte leukemia and cirrhosis developed severe thrombocytopenia within 6 days of starting carboplatin [platelet count 15,000/µL, bilirubin 5.6 mg/dL, AST 4690 U/L, Alk P 150 U/L] and death from multiorgan failure 10 days later; autopsy showed cirrhosis and marked centrolobular necrosis).
- Tran A, Housset C, Boboc B, Tourani J-M, Carnot F, Berthelot P. Etoposide (VP 16-213) induced hepatitis: report of three cases following standard dose treatments. J Hepatol. 1991;12:36–9. [PubMed: 2007774](3 patients, ages 52 to 73 years, developed jaundice 1-5 months after starting etoposide with several other cyclic antineoplastic agents including cisplatin and cyclophosphamide in two [bilirubin 4.2-13.0 mg/dL, ALT 790-2270 U/L, Alk P 181-280 U/L], resolving in 4-10 weeks and no recurrence on a similar regimen without etoposide in one patient).
- Bishop JF. Current experience with high-dose carboplatin therapy. Semin Oncol. 1992;19:150–4. [PubMed: 1411626](Dose limiting toxicities of high dose carboplatin followed by bone marrow transplantation were reversible cholestatic hepatitis, renal dysfunction and ototoxicity).
- Ayash LJ, Elias A, Wheeler C, Reich E, Schwartz G, Mazanet R, Tepler I, et al. Double dose-intensive chemotherapy with autologous marrow and peripheral-blood progenitor-cell support for metastatic breast cancer: a feasibility study. J Clin Oncol. 1994;12:37–44. [PubMed: 7505807](Among 29 men with advanced testicular cancer receiving carboplatin and etoposide, 6 [21%] developed "elevation of liver function tests" but no mention of veno-occlusive disease).
- Hartmann JT, Lipp H-P. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. [PubMed: 12783586](Review of pharmacology, mechanism of action, adverse effects and tolerance of platinum containing alkylating agents; "Mild reversible increases in liver function tests can occur in patients who have received platinum compounds. However, the platinum compounds are generally not classified as hepatotoxic drugs").
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–60. [PMC free article: PMC2914093] [PubMed: 20373370](Review of liver complications of bone marrow [hematopoietic cell] transplantation, which have become less frequent with better understanding of their causes and means of prevention; the rate of SOS has decreased because of avoidance of more aggressive ablative therapies [total body irradiation and high doses of cyclophosphamide] and better understanding of pharmacokinetics of the alkylating agents).
- Laemmle A, Hahn D, Hu L, Rüfenacht V, Gautschi M, Leibundgut K, Nuoffer JM, et al. Fatal hyperammonemia and carbamoyl phosphate synthetase 1 (CPS1) deficiency following high-dose chemotherapy and autologous hematopoietic stem cell transplantation. Mol Genet Metab. 2015;114:438–44. [PubMed: 25639153](2 year old boy with neuroblastoma developed severe, refractory hyperammonemia with normal liver tests and liver histology 7 days after hematopoietic cell transplantation and myeloablative therapy with melphalan etoposide and carboplatin, liver tissue revealing virtually no CPS1 activity or protein despite relatively normal mRNA levels and no mutations in the cDNA of the CPS1 gene; in vitro assays demonstrating post-transcription inhibition of CPS1 by the combination of carboplatin and etoposide).
- Tareen SA, Rodriguez B, Bolos D. Retrospective analysis of HER2+ breast cancer outcomes at a county hospital: do published outcomes hold up in the real world? Cureus. 2020;12:e7937. [PMC free article: PMC7266367] [PubMed: 32499977](Among 24 patients with HER2+ breast cancer treated in community practice with a standard regimen of docetaxel, carboplatin, trastuzumab and pertuzumab, clinical response rates were similar to those reported from large registration trials of this regimen [50% vs 52%], but side effects were more common including liver test abnormalities [38% vs 4%] perhaps due to the larger proportion of Hispanic minorities in the treated population [57%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Platinum Coordination Complexes.[LiverTox: Clinical and Researc...]Review Platinum Coordination Complexes.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cisplatin.[LiverTox: Clinical and Researc...]Review Cisplatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Oxaliplatin.[LiverTox: Clinical and Researc...]Review Oxaliplatin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- In vitro phase II comparison of the cytotoxicity of a novel platinum analog, nedaplatin (254-S), with that of cisplatin and carboplatin against fresh, human ovarian cancers.[Cancer Chemother Pharmacol. 1997]In vitro phase II comparison of the cytotoxicity of a novel platinum analog, nedaplatin (254-S), with that of cisplatin and carboplatin against fresh, human ovarian cancers.Alberts DS, Fanta PT, Running KL, Adair LP Jr, Garcia DJ, Liu-Stevens R, Salmon SE. Cancer Chemother Pharmacol. 1997; 39(6):493-7.
- Review Carboplatin in the treatment of squamous cell head and neck cancers.[Semin Oncol. 1992]Review Carboplatin in the treatment of squamous cell head and neck cancers.Aisner J, Sinibaldi V, Eisenberger M. Semin Oncol. 1992 Feb; 19(1 Suppl 2):60-5.
- Carboplatin - LiverToxCarboplatin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...