NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Digitalis and its derivatives such as digoxin and digitoxin are cardiac glycosides used typically in the therapy of congestive heart failure and atrial fibrillation. The cardiac glycosides have not been linked to serum enzyme elevations during therapy or with instances of clinically apparent liver injury.
Background
Digitalis (dij" i tal' is), digoxin (di jox' in) and digitoxin (dij" i tox' in) are cardiac glycosides that enhance myocardial contractility, probably by increasing levels of myocardial cytosolic calcium because of inhibition of sodium-potassium ATPase. Originally derived from the purple foxglove flower (Digitalis purpurea), the cardiac glycosides have been used in clinical medicine for more than two centuries. Once used as first-line agents for congestive heart failure and atrial fibrillation, the cardiac glycosides have been replaced by agents that are better tolerated and have been shown to improve long term survival such as the ACE inhibitors and beta-blockers. The cardiac glycosides are now reserved largely for patients with left ventricular systolic dysfunction and atrial fibrillation or for patients with congestive heart failure in sinus rhythm and residual symptoms despite maximal alternative therapy. Digitalis has been available for over a century. Digoxin, derived from Digitalis lanatus, was introduced as having more reliable pharmacokinetics and remains the major form of cardiac glycosides used today. The cardiac glycosides are approved treatment of mild-to-moderate congestive heart failure and for control of the ventricular response rate in patients with atrial fibrillation. Digoxin is available in tablets of ranging from 0.0625 to 0.5 mg generically and under the brand name Lanoxin. The typical dose is 0.125 to 0.25 mg daily, but dosing is often individualized because of tolerance and effect. Digoxin is also available in solution for intravenous administration as well as an oral solution. The cardiac glycosides have many side effects that are largely dose related and require careful monitoring of drug levels. The most common side effects include dizziness, fatigue, headache, anxiety, gastrointestinal upset, change in taste and blurred vision. Severe side effects include seizures and coma, heart block, atrial and ventricular arrhythmias and sudden cardiac death.
Hepatotoxicity
In clinical trials, digoxin was not associated with serum aminotransferase or alkaline phosphatase elevations. Despite widespread use for many decades, the cardiac glycosides have not been implicated in cases of clinically apparent liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The safety of the cardiac glycosides is probably due to the low doses used (less than 1 mg daily) and the serious cardiac and neurologic complications that accompany use of higher doses that might be required for liver injury. The mechanism by which digoxin or digitalis might cause liver injury is unknown. Digoxin is minimally metabolized in the liver and does not materially affect the activity of cytochrome P450 enzymes.
Drug Class: Cardiac Glycosides
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Digoxin – Lanoxin®
DRUG CLASS
Cardiac Glycosides
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Digoxin | 20830-75-5 | C41-H64-O14 |
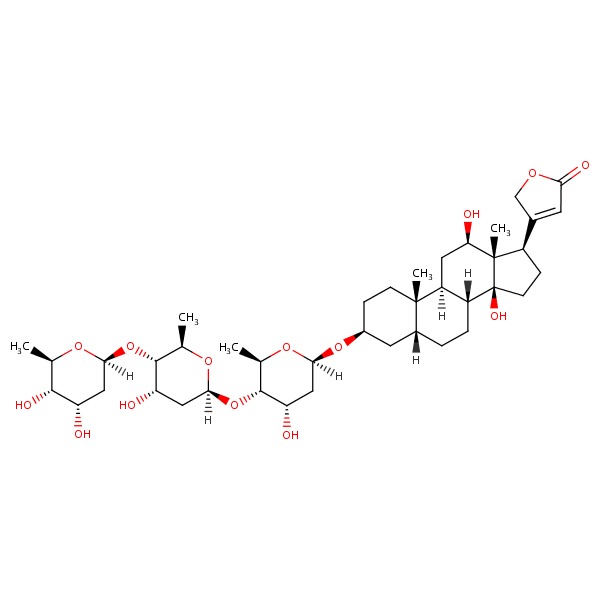 |
| Digitoxin | 71-63-6 | C41-H64-O13 |
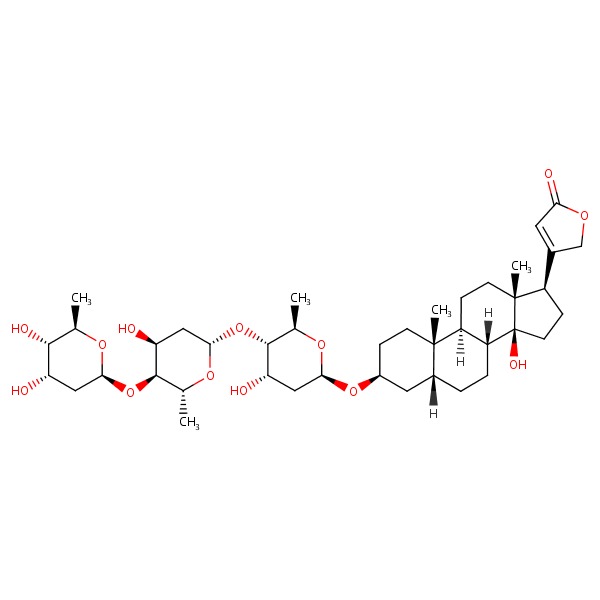 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 January 2018
- Zimmerman HJ. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity published in 1999 does not discuss the cardiac glycosides).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity of cardiovascular agents does not discuss the cardiac glycosides).
- Lucena MI, Stephens C, García-Cortés M, Andrade RJ. Hepatotoxic potential. Causality assessment. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 290.(Review of the potential of different medications to cause liver injury mentions that there is little evidence to implicate digoxin in toxic liver damage despite years of use).
- Maron BA, Rocco TP. Cardiac glycosides. Pharmacology of congestive heart failure. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 801-4.(Textbook of pharmacology and therapeutics).
- Hochrein H. [Letter: Liver toxicity of digitalis glycosides]. Dtsch Med Wochenschr 1975 Apr 18; 100 (16): 913. German. [PubMed: 1122858](Answer to the question of whether digitalis can cause liver injury mentions that there is no evidence that the cardiac glycosides cause liver injury even at high doses, and that the major concern would be the possibility of increased risk of cardiac complications of glycosides in patients with advanced liver disease).
- Bigger JT Jr. Digitalis toxicity. J Clin Pharmacol 1985; 25: 514-21. [PubMed: 3905880](Review of the toxicity of cardiac glycosides with conventional as well as excessive doses, focusing upon neurologic [confusion, stupor, coma, seizures] and cardiac side effects [brady- and tachy-arrhythmias] as well as evolving data suggesting a decrease in survival when used after myocardial infarction).
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525-33. [PubMed: 9036306](Among 6800 patients with congestive heart failure treated with digoxin or placebo with an average follow up of 3 years, survival rates were similar in the two groups, but hospitalizations for worsening heart failure were less; no mention of ALT elevations or hepatotoxicity).
- Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Schmitt S, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol 2014; 64: 660-8. [PMC free article: PMC4405246] [PubMed: 25125296](Retrospective analysis of healthcare data from the VA system identified 122,465 patients with atrial fibrillation, 23% of whom received digoxin; mortality was higher with digoxin use, but rates of death were not reported).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a cardiac glycoside).
- Drugs for chronic heart failure. Med Lett Drugs Ther 2015; 57 (1460): 9-13. [PubMed: 25581106](Concise review of drugs approved for use in treatment of congestive heart failure mentions that digoxin can improve symptoms of congestive heart failure but does not prolong survival, the most common adverse events being conduction disturbances, cardiac arrhythmias, nausea, vomiting, confusion and visual disturbances).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure.[Am Heart J. 1947]Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure.BATTERMAN RC, DE GRAFF AC. Am Heart J. 1947 Nov; 34(5):663-73.
- Review Treatment of congestive heart failure--current status of use of digitoxin.[Eur J Clin Invest. 2001]Review Treatment of congestive heart failure--current status of use of digitoxin.Belz GG, Breithaupt-Grögler K, Osowski U. Eur J Clin Invest. 2001; 31 Suppl 2:10-7.
- [MAINTENANCE DIGITALIS TREATMENT OF CHRONIC CARDIAC INSUFFICIENCY].[Acta Clin Belg. 1963][MAINTENANCE DIGITALIS TREATMENT OF CHRONIC CARDIAC INSUFFICIENCY].MAURICE P, SCEBAT L. Acta Clin Belg. 1963; 18:315-25.
- [Enteral resorption of digitoxin and gitoxin].[Arzneimittelforschung. 1951][Enteral resorption of digitoxin and gitoxin].HOTOVY R. Arzneimittelforschung. 1951 Jul; 1(4):160-4.
- Review Current status of cardiac glycoside drug interactions.[Clin Pharm. 1985]Review Current status of cardiac glycoside drug interactions.Hooymans PM, Merkus FW. Clin Pharm. 1985 Jul-Aug; 4(4):404-13.
- Cardiac Glycosides - LiverToxCardiac Glycosides - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...