NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bisoprolol is a cardioselective beta-blocker used in the treatment of hypertension. Bisoprolol has not been linked to instances of clinically apparent drug induced liver injury.
Background
Bisoprolol (bis" oh proe' lol) is considered a “selective” beta-adrenergic receptor blocker in that it has potent activity against beta-1 adrenergic receptors which are found in cardiac muscle, but has little or no activity against beta-2 adrenergic receptors found on bronchial and vascular smooth muscle. Bisoprolol was approved for use in the United States in 1992 and is currently widely used in the therapy of hypertension alone or in combination with other agents. At present, more than 4 million prescriptions for bisoprolol are filled yearly. Bisoprolol is available in 5 and 10 mg tablets in generic forms as well as under the trade name of Zebeta. Bisoprolol is also available in fixed combinations with hydrochlorthiazide (Ziac and other generic forms). The usual initial oral dose of bisoprolol in adults is 2.5 to 5 mg once daily, with subsequent adjustment based upon clinical response and tolerance, the usual maintenance dosage being 5 to 20 once mg daily. Common side effects include bradycardia, hypotension, fatigue, dizziness, depression, insomnia, memory loss and impotence. At high doses, bisoprolol is less cardioselective and can induced acute bronchospasm. As with all beta-blockers, sudden withdrawal can trigger rebound hypertension.
Hepatotoxicity
Bisoprolol therapy has been associated with a low rate of mild-to-moderate elevations of serum aminotransferase levels which are usually asymptomatic and transient and resolve even with continuation of therapy. There have been no well documented cases of clinically apparent, acute liver injury attributable to bisoprolol. Thus, hepatotoxicity due to bisoprolol must be very rare, if it occurs at all. Most commonly used beta-blockers have been linked to rare instances of clinically apparent liver injury, typically with onset within 2 to 12 weeks, a hepatocellular pattern of liver enzyme elevations, rapid recovery upon withdrawal, and little evidence of hypersensitivity (rash, fever, eosinophilia) or autoantibody formation.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of drug induced liver injury from beta-blockers such as bisoprolol is not known. Bisoprolol is extensively metabolized by the liver and excreted as inactive metabolites. The few rare cases reported were likely idiosyncratic.
Outcome and Management
The severity of liver injury due to beta-blockers ranges from mild serum aminotransferase elevations to acute hepatitis with jaundice. In large case series of drug induced liver injury and acute liver failure due to medications, bisoprolol has not been listed as a potential cause. There is little information about cross reactivity among the beta-blockers to hepatic injury. Switching from a beta-blocker that has caused acute liver injury to another should be done with caution and active monitoring.
References to the safety and potential hepatotoxicity of bisoprolol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bisoprolol – Generic, Zebeta®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
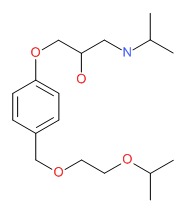
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bisoprolol | 66722-44-9 | C18-H31-N-O4 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review [Bisoprolol: opportunities in the treatment of hypertension].[Kardiologiia. 2012]Review [Bisoprolol: opportunities in the treatment of hypertension].Minushkina LO. Kardiologiia. 2012; 52(6):80-5.
- Bisoprolol.[StatPearls. 2024]Bisoprolol.Bazroon AA, Alrashidi NF. StatPearls. 2024 Jan
- Bisoprolol--another cardioselective beta blocker.[Drug Ther Bull. 1989]Bisoprolol--another cardioselective beta blocker.. Drug Ther Bull. 1989 Jul 10; 27(14):55-6.
- Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS).[Cardiovasc Drugs Ther. 2003]Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS).Czuriga I, Riecansky I, Bodnar J, Fulop T, Kruzsicz V, Kristof E, Edes I, NEBIS Investigators, NEBIS Investigators Group. Cardiovasc Drugs Ther. 2003 May; 17(3):257-63.
- Review [Bipressil® : first single-pill combination of bisoprolol and perindopril arginine].[Rev Med Liege. 2017]Review [Bipressil® : first single-pill combination of bisoprolol and perindopril arginine].Gach O, Falque B, Canivet A, Krzesinski F, Krzesinski JM, Lancellotti P. Rev Med Liege. 2017 May; 72(5):260-265.
- Bisoprolol - LiverToxBisoprolol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...