NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The bisphosphonates are pyrophosphate analogues that become incorporated into bone matrix and suppress osteoclastic activity, thereby reducing bone turnover and increasing bone mass, which makes them valuable agents for the prevention and therapy of osteoporosis. Therapy with the bisphosphonates has been associated with a low rate of serum enzyme elevations during therapy and has been linked to rare instances of clinically apparent liver injury.
Background
Bisphosphonates are pyrophosphate analogues that have two phosphonate groups attached to a central carbon atom that replaces the oxygen present in pyrophosphate. The bisphosphonates bind calcium and are rapidly taken up in bone matrix where they suppress osteoclastic activity and change the balance between bone resorption and bone formation, thus increasing bone mass. The bisphosphonates have been shown to be effective in treating malignant hypercalcemia and in preventing and treating osteoporosis. Six bisphosphonates have been approved for use in the United States (pronunciation and year of approval given in parentheses) and they differ in formulation, recommended dose regimen, spectrum of activity and clinical indications.
• Alendronate (a len' droe nate) (1995) is available in tablets of 5 and 10 mg for daily use, 35, 40 and 70 mg (with and without vitamin D) for weekly use, and as a suspension for oral use in several generic forms and under the brand name Fosamax. Indications include prevention and treatment of osteoporosis and treatment of Paget disease of bone.
• Etidronate (e" ti droe' nate) (1977) is available in tablets of 200 and 400 mg for daily use in generic forms and under the trade name Didronel. Indications include Paget disease of bone and heterotopic ossification, but it has also been used off label for therapy of osteoporosis.
• Ibandronate (eye" ban droe' nate) (2003) is available in tablets of 2.5 mg for daily and 150 mg for monthly use and as an intravenous formulation under the trade name Boniva. Indications include prevention and treatment of osteoporosis.
• Pamidronate (pam" i droe' nate) (1991) is available as an intravenous formulation generically and under the trade name Aredia. Indications include hypercalcemia of malignancy, multiple myeloma and Paget disease of bone.
• Risedronate (ris" e droe' nate) (1998) is available in tablets of 5 mg for daily use, 30 and 35 mg for weekly use, and 75 and 150 mg for monthly use in generic forms and under the trade name Actonel. Indications include osteoporosis and Paget disease of bone.
• Zoledronic acid (zoe" le droe' nate) (2001) is available as several intravenous formulations generically and under the brand names Zometa and Reclast. Indications and dosage vary by preparation, but include prevention and treatment of osteoporosis, Paget disease of bone, hypercalcemia of malignancy and multiple myeloma.
The side effects of the bisphosphonates vary by route of administration, but are largely class specific. The oral formulations are generally well tolerated, but are recommended to be given on an empty stomach and with care that they enter the stomach (by drinking water and remaining upright) to avoid esophageal irritation and potential ulceration. Common side effects of oral formulations include headache, abdominal discomfort, dyspepsia, nausea and hypocalcemia. The intravenous formulations of the bisphosphonates can be associated with local infusion reactions and in an acute phase reaction in up to 30% of patients. This is characterized by a flu-like syndrome primarily with the initial infusion. Symptoms arise within 10 to 20 hours after the infusion and are accompanied by increases in C reactive protein, decreases in serum zinc and, in some instances, minor elevations in serum enzymes several days later. Severe side effects of the bisphosphanates are rare, but have included esophageal ulcer, gastrointestinal bleeding, atrial fibrillation and, with long-term treatment, osteonecrosis of the jaw and atypical femoral fractures.
Hepatotoxicity
In most large prospective trials, the bisphosphonates were associated with only rare and isolated instances of serum enzyme elevations and no cases of clinically apparent liver injury. Since their general availability and wide scale use, however, there have been occasional publications reporting clinically apparent acute liver injury due to the more commonly used bisphosphonates (alendronate, ibandronate, risedronate, zoledronate), some of which were accompanied by mild jaundice. The time to onset ranged from 2 to 6 months or more, and patients typically presented with abdominal discomfort and nausea, sometimes followed by jaundice. The pattern of serum enzyme elevations was hepatocellular and liver histology showed an acute toxic hepatitis. Immunoallergic features (fever, rash, eosinophilia) and autoantibodies were uncommon. Most cases were mild-to-moderate in severity and most published cases resolved with drug discontinuation, although full recovery was not always prompt.
In addtion, the bisphosphonates given as intravenous infusions (zoledronate, ibandronate, pamidronate) have been associated with rare instances of mild hypersensitivity reactions with rash and fever which may be accompanied by transient and mild serum enzyme elevations without jaundice typically in association with an acute phase reaction occurring with the initial dose. In some cases, infusions can be tolerated using premedication with glucocorticoids or antihistamines. With repeated doses, these reactions become less severe and may disappear. No instances of acute liver failure or chronic liver disease have been convincingly linked to use of the bisphosphonates.
Likelihood scores:
Alendronate: C (probable rare cause of clinically apparent liver injury).
Etidronate: E* (unproven but suspected rare cause of clinically apparent liver injury).
Ibandronate: D (possible rare cause of clinically apparent liver injury).
Pamidronate: E* (unproven but suspected rare cause of clinically apparent liver injury). Risedronate: D (possible rare cause of clinically apparent liver injury).
Zoledronic acid: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The bisphosphonates are taken up by bone matrix and rapidly cleared from the serum by renal excretion. Hepatic metabolism is minimal, and thus it is somewhat surprising that they can be associated with hepatic injury. The mechanism of injury is likely to be metabolic idiosyncrasy as immunoallergic features are not typical.
Outcome and Management
The clinically apparent acute liver injury attributed to bisphosphonates has been mild-to-moderate in severity without published instances of acute liver failure or chronic liver disease. The possibility of cross reactivity of the hepatic injury among the various bisphosphonates has not been studied, nor is there published experience with rechallenge using the same bisphosphonates. Cross reactivity to such injury should be assumed and switching to another agent done with caution and careful monitoring.
References to the safety and potential hepatotoxicity of all six agents are given at the end of this Overview section.
Drug Class: Osteoporosis Agents, Bone Resorption Inhibitors
CASE REPORTS
Case 1. Asymptomatic rise in serum aminotransferase levels during alendronate therapy.
[Modified from: de La Serna Higuera C, Pérez Villoria A, Rodríguez Gómez S, Martínez Moreno J, Betancourt González A, Martín Arribas M. [Alendronate-induced hepatocellular lesion]. Gastroenterol Hepatol 2001; 24: 244-6. Spanish. PubMed Citation]
A 76 year old woman with osteoporosis was found to have abnormal serum aminotransferase levels on routine testing done 3 months after starting alendronate (10 mg daily). She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Her other medical conditions included hypertension, but she was not taking any other medications. She denied symptoms of liver disease and physical examination was normal without rash, fever, or hepatic enlargement or tenderness. Serum ALT levels were elevated with ALT 484 U/L, AST 368 U/L, and GGT 118 U/L, but normal alkaline phosphatase, bilirubin and prothrombin time (Table). Tests for hepatitis A, B and C (including HCV RNA) were negative as were routine autoantibodies. Abdominal ultrasound showed no abnormalities of the liver or biliary tree. Alendronate was continued and she was monitored. Two months later, serum aminotransferase levels had risen further and alendronate was stopped. Liver test abnormalities subsequently improved and serum aminotransferase levels were largely normal six weeks later.
Key Points
| Medication: | Alendronate (10 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=12) |
| Severity: | 1+ (serum enzyme elevations without jaundice or symptoms) |
| Latency: | 3 months |
| Recovery: | 6 weeks |
| Other medications: | None |
Laboratory Values
Comment
The absence of any other cause of liver injury combined with worsening abnormalities while alendronate was continued, followed by prompt improvement on stopping, is reasonably good evidence that the bisphosphonate was the cause of the liver test abnormalities. The patient was never jaundiced and was minimally symptomatic.
Case 2. Asymptomatic rise in serum aminotransferase levels after initial dose of zoledronic acid.
[Modified from: Jiang Y, Fu Y, Xing XP, Li M, Wang O, Xia WB, Meng XW. Zoledronic acid-induced hepatotoxicity relieved after subsequent infusions in a Chinese woman with glucocorticoid-induced osteoporosis. Eur J Med Res 2015; 20: 68. PubMed Citation]
A 50 year old woman with glucocorticoid-induced osteoporosis developed fever, myalgia and arthralgia a few hours after an initial infusion of zoledronic acid and 3 days later was found to have abnormal serum aminotransferase levels which had been normal before treatment (Table). She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. She had a history of Behcet disease for which she was treated with corticosteroids in varying doses for the previous 12 years. Her other medical conditions included diabetes for which she was taking metformin. Osteoporosis was diagnosed when she developed back pain and vertebral fractors after a fall. She did not improve with modification of her corticosteroid dose, calcitriol and elemental calcium and zoledronic acid was given. Her flu-like symptroms developed a few hours after the initial infusion, but resolved rapidly. Routine blood tests 3 days later showed serum enzyme elevations without jaundice or symptoms. These resolved within the following two weeks. Serum bilirubin and alkaline phosphatase levels remained normal. She received a second influsion of zoledronic acid one year later and again had mild fever and arthralgia as well as minimal ALT elevations 3 days later. A third infusion of zolendronic acid the following year was well tolerated, without fever or myalgia and will no subsequent rise in serum enzymes.
Key Points
| Medication: | Zoledronic acid (5 mg intravenously once yearly) |
|---|---|
| Pattern: | Hepatocellular (R=~6.4) |
| Severity: | 1+ (serum enzyme elevations without jaundice or symptoms) |
| Latency: | 3 days |
| Recovery: | ~2 weeks |
| Other medications: | Metformin, methylprednisolone, glucurolactone, calcitriol |
Laboratory Values
| Time After Administration | ALT (U/L) | Alk P (U/L) | GGT (U/L) | Bilirubin (mg/dL) |
|---|---|---|---|---|
| Immediately before | 33 | 77 | 45 | 0.6 |
| 1st infusion | Zoledronic acid (5 mg iv), followed by fever and arthralgia | |||
| 3 days | 254 | 135 | 0.4 | |
| 6 days | 126 | 118 | 112 | 0.5 |
| 9 days | 66 | 0.7 | ||
| 1 year | 28 | 44 | 30 | 0.6 |
| 2nd infusion | Zolendronic acid (5 mg iv), followed by fever and arthralgia | |||
| 3 days (after 2nd) | 70 | 46 | 0.3 | |
| 6 days (after 2nd) | 48 | 58 | 43 | 0.4 |
| 2 years | 24 | 51 | 27 | 0.5 |
| 3rd infusion | Zolendronic acid (5 mg iv), followed by no fever or pain | |||
| 3 days (after 3rd) | 31 | 55 | 33 | 0.4 |
| Normal Values | <40 | <120 | <67 | <1.2 |
Comment
Intravenous formulations of bisphosphanates are associated with an acute phase reaction in approximately one-third of patients. Flu-like symptoms of fever, fatigue, muscle and joint aches, gastrointestinal upset and nausea arise within hours of the infusion, but are generally mild and transient, resolving within 12 to 48 hours. Monitoring of laboratory tests demonstrates rises in acute phase reactants 1-3 days later and this may be accompanied by mild elevations in serum aminotranferase levels, but generally without symptoms or signs of liver disease and little change in alkaline phosphatase or serum bilirubin levels. The syndrome is most prominent with the first infusion and can be ameliorated by use of NSAIDs or, in more severe cases, corticosteroids.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Alendronate – Generic, Fosamax®
Etidronate – Generic, Didronel®
Ibandronate – Generic, Boniva®
Pamidronate – Generic, Aredia®
Risedronate – Generic, Actonel®
Zoledronic Acid – Reclast®, Zometa®
DRUG CLASS
Osteoporosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Alendronate | 121268-17-5 | C4-H13-N-O7-P2.3H2-O.Na |
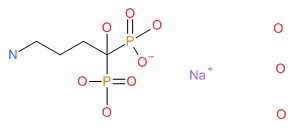 |
| Etidronate | 2809-21-4 | C2-H8-O7-P2 |
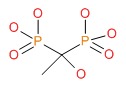 |
| Ibandronate | 114084-78-5 | C9-H23-N-O7-P2 |
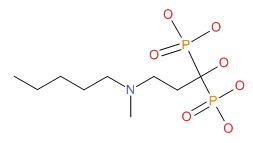 |
| Pamidronate | 40391-99-9 | C3-H11-N-O7-P2 |
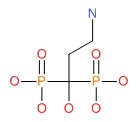 |
| Risedronate | 105462-24-6 | C7-H11-N-O7-P2 |
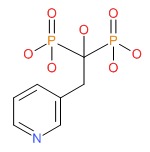 |
| Zoledronic Acid | 118072-93-8 | C5-H10-N2-O7-P2 |
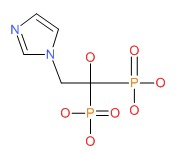 |
ANNOTATED BIBLIOGRAPHY
References updated: 14 June 2018
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 543.(Review of hepatotoxicity published in 1999; bisphosphonates are not discussed).
- Friedman PA. Agents affecting mineral ion homeostasis and bone turnover. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1275-1306.(Textbook of pharmacology and therapeutics).
- Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int 1987; 41: 326-31. [PubMed: 3124942](Among 52 patients with hyperparathyroidism or Paget disease treated with intravenous amino-derived bisphosphonates, a dose dependent acute phase reaction arose 10-20 hours after the initial infusion with fever, lymphopenia, fall in serum zinc levels and rise in C-reactive protein, resolving spontaneously in 3-4 days).
- Nussbaum SR, Warrell RP Jr, Rude R, Glusman J, Bilezikian JP, Stewart AF, Stepanavage M, et al. Dose-response study of alendronate sodium for the treatment of cancer-associated hypercalcemia. J Clin Oncol 1993; 11: 1618-23. [PubMed: 8336198](Multicenter randomized trial of 5 dose regimens of intravenous alendronate in 59 patients with malignant hypercalcemia; 8 patients had transient, asymptomatic aminotransferase elevations, which were reversible in all instances).
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333: 1437-43. [PubMed: 7477143](Controlled trial of daily alendronate vs placebo for 2 years in 994 women with osteoporosis; "Alendronate was generally well tolerated, with no greater clinical or laboratory evidence of adverse effects than with placebo").
- Chesnut CH 3rd, McClung MR, Ensrud KE, Bell NH, Genant HK, Harris ST, Singer FR, et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med 1995; 99: 144-52. [PubMed: 7625419](Controlled trial of multiple doses of daily oral alendronate vs placebo in 188 women with osteopenia; higher doses were associated with gastrointestinal intolerance, rash and "transient, small increases" in ALT [< twice ULN], which resolved spontaneously or with drug discontinuation and no clinically apparent liver injury).
- Conte PF, Latreille J, Mauriac L, Calabresi F, Santos R, Campos D, Bonneterre J, et al. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. J Clin Oncol 1996; 14: 2552-9. [PubMed: 8823335](Controlled trial of intravenous pamidronate in 295 women undergoing chemotherapy for breast cancer; during 1598 infusions adverse events included transient elevation in AST [<3 times ULN] in one patient).
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535-41. [PubMed: 8950879](Controlled trial of 24 months of daily alendronate vs placebo in 2027 women with previous history of vertebral fracture, "the rates of all adverse events and gastrointestinal adverse events...did not differ by study treatment").
- Lieverse RJ. Hepatitis after alendronate. Neth J Med 1998; 53: 271-2. [PubMed: 9883006](77 year old woman developed weight loss and jaundice 6 months after starting alendronate [bilirubin 16.9 mg/dL, ALT 25 times ULN, Alk P 3 times ULN], resolving within 3 months of stopping).
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280: 2077-82. [PubMed: 9875874](Controlled trial of oral alendronate vs placebo in 4432 women with osteopenia for an average of 4.2 years; there was no differences in rates of adverse events between the two groups).
- Dooley M, Balfour JA. Ibandronate. Drugs 1999; 57: 101-8; discussion 109-10. [PubMed: 9951955](Review of chemistry, pharmacology, efficacy and safety of ibandronate; "no significant changes were observed in laboratory parameters ... or liver and kidney function in ibandronate recipients").
- Laitinen K, Taube T. Clodronate as a cause of aminotransferase elevation. Osteoporos Int 1999; 10: 120-2. [PubMed: 10501791](Controlled trial of 5 different dose regimens of clodronate or placebo in 610 postmenopausal women with osteopenia, showed dose related average increase in ALT and AST levels, with increase above normal in 18% of clodronate treated vs 7% [1 of 14] of controls).
- Pols HA, Felsenberg D, Hanley DA, Stepán J, Muñoz-Torres M, Wilkin TJ, Qin-sheng G, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 1999; 9: 461-8. [PubMed: 10550467](Controlled trial of 1 year course of daily oral alendronate vs placebo in 1908 women with osteopenia found no differences in rates of adverse events between the two groups; ALT results not mentioned).
- Peter C, Rodan GA. Preclinical safety profile of alendronate. Int J Clin Pract Suppl 1999; 101: 3-8. [PubMed: 12669734](Acute and chronic toxicity studies of alendronate in animals found nephrotoxicity and decreases in calcium and phosphate with very high doses, but no evidence of toxicity at standard human therapeutic doses).
- Watts N, Freedholm D, Daifotis A. The clinical tolerability profile of alendronate. Int J Clin Pract Suppl 1999; 101:51-61. [PubMed: 12669741](Overview of side effects of alendronate in large prelicensure, controlled trials in more than 17,000 women; overall, laboratory adverse events were more common with placebo [9.3%] than alendronate [6.3%] and there were no serious laboratory adverse events; hepatotoxicity and ALT elevations were not discussed).
- Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000; 343: 604-10. [PubMed: 10979796](Controlled trial of daily oral alendronate vs placebo in 241 men with osteoporosis; one man on alendronate stopped therapy because of high AST levels, but details not given).
- Halabe A, Lifschitz BM, Azuri J. Liver damage due to alendronate. N Engl J Med 2000; 343: 365-6. [PubMed: 10928896](71 year old woman developed asymptomatic elevations in serum enzymes 2 months after starting alendronate [ALT 163 rising to 447 U/L, Alk P 151 rising to 224 U/L], abnormalities resolving within 4 months of stopping).
- de La Serna Higuera C, Pérez Villoria A, Rodríguez Gómez S, Martínez Moreno J, Betancourt González A, Martín Arribas M. [Alendronate-induced hepatocellular lesion]. Gastroenterol Hepatol 2001; 24: 244-6. Spanish. [PubMed: 11412594](76 year old woman developed elevated ALT levels 3 months after starting alendronate [ALT 484 U/L, Alk P normal], resolving within 6 weeks of stopping: Case 1).
- Carrère C, Duval JL, Godard B, De Jaureguiberry JP, Ciribilli JM. [Severe acute hepatitis induced by alendronate]. Gastroenterol Clin Biol 2002; 26: 179-80. French. [PubMed: 11938071](71 year old woman developed jaundice 4 months after starting alendronate [bilirubin 21.7 mg/dL, ALT 25 times ULN, Alk P 1.1 times ULN], resolving within 3 months of stopping).
- Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yates J, et al. Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc 2002; 77: 1044-52. [PubMed: 12374248](Controlled trial of 12 week courses of once weekly alendronate vs placebo in 450 men and women with osteoporosis; there were no differences in rates of side effects between the two groups; no mention of ALT levels).
- Guañabens N, Parés A, Ros I, Alvarez L, Pons F, Caballería L, Monegal A, et al. Alendronate is more effective than etidronate for increasing bone mass in osteopenic patients with primary biliary cirrhosis. Am J Gastroenterol 2003; 98: 2268-74. [PubMed: 14572578](32 women with primary biliary cirrhosis were treated with alendronate or etidronate for 14 days every 3 months for up to 2 years; there were no significant changes in liver biochemical test results).
- Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, Melton ME. Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 2004; 20: 699-705. [PubMed: 15140336](Analysis of adverse events in 449 women and men with osteoporosis treated with once weekly alendronate vs placebo for 12 weeks; upper gastrointestinal side effects occurred at similar rates in the two groups [9.8% vs 9.4%], but results of ALT values and other laboratory tests were not mentioned).
- Chesnut III CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, et al.; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19: 1241-9. [PubMed: 15231010](Controlled trial of oral ibandronate vs placebo in 2496 women with osteopenia for up to 3 years; there were no differences in frequency of side effects between ibandronate and placebo treated groups and no "clinically relevant changes in laboratory parameters").
- Phillips MB. Risedronate-induced hepatitis. Am J Med 2007; 120: e1-2. [PubMed: 17349419](81 year old woman with osteoporosis developed rise in ALT after 3 years of risedronate therapy, resolving slowing upon stopping; few details given).
- Yanik B, Turkay C, Atalar H. Hepatotoxicity induced by alendronate therapy. Osteoporos Int 2007; 18: 829-31. [PubMed: 17226065](47 year old woman developed abdominal pain 2 months after starting alendronate [bilirubin not given, ALT 46 rising to 114 U/L, GGT 30 rising to 84 U/L], resolving within 3 months of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to a bisphosphonate).
- Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009; 8: 96-110. [PubMed: 19570737](Review of side effects of bisphosphonates mentions that a few cases of hepatitis have been reported).
- Polyzos SA, Kountouras J, Anastasilakis AD, Litsas I, Kita M, Arsos G, Moralidis E, Terpos E. Zoledronic acid-induced transient hepatotoxicity in a patient effectively treated for Paget's disease of bone. Osteoporos Int 2011; 22: 363-7. [PubMed: 20407889](53 year old woman with Paget disease developed mild flu-like symptoms and serum enzyme elevations within a day of zoledronic acid infusion [bilirubin 0.4 mg/dL ALT rising from 19 to 127 U/L, GGT from 11 to 74 U/L], falling to normal within two weeks).
- Rudic JS, Giljaca V, Krstic MN, Bjelakovic G, Gluud C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst Rev 2011; 7 (12): CD009144. [PubMed: 22161446](Systematic review of studies on safety and efficacy of bisphosphonates in primary biliary cirrhosis found no evidence that they adversely affected the underlying liver disease).
- Anastasilakis AD, Polyzos SA, Makras P, Sakellariou GT, Bisbinas I, Gkiomisi A, Delaroudis S, et al. Acute phase response following intravenous zoledronate in postmenopausal women with low bone mass. Bone 2012; 50: 1130-4. [PubMed: 22366634](Among 51 women with osteoporosis given a single intravenous dose of zoledronic acid, 28 [55%] developed an acute phase reaction starting 18 hours after the infusion and lasting for 43 hours with fever, muscle and joint pain, fatigue and headache with decrease in lymphocytes and increase in CRP, IL-1, IL-6 and TNF, but no change in mean ALT, AST or Alk P at 48 hours).
- Kaemmerer D, Schmidt B, Lehmann G, Wolf G, Settmacher U, Hommann M. Treatment of bone loss in patients with chronic liver disease awaiting liver transplantation. Transplant Res 2012; 1: 7. [PMC free article: PMC3560973] [PubMed: 23369371](Among 31 patients with osteoporosis undergoing liver transplantation treated with oral ibandronate, calcium and vitamin D for one year, none had worsening bone mineral density scores, and mean scores increased by 13-24% at the lumbar spine and 3-5% at the femoral neck; no mention of adverse events).
- Goossens N, Spahr L, Rubbia-Brandt L. Severe immune-mediated drug-induced liver injury linked to ibandronate: A case report. J Hepatol 2013 59; 1139-42. [PubMed: 23770145](61 year old woman with lupus erythematosus developed fatigue 4 months after starting monthly injections of ibandronate and 12 months after starting atorvastatin [bilirubin 8.0 mg/dL, ALT 1684 U/L, Alk P 208 U/L, ANA 1:640, SMA 1:160, IgG 2,850 mg/dL], responding to prednisone, but relapsing when they were stopped, ultimately resolving after ursodiol therapy).
- Guañabens N, Monegal A, Cerdá D, Muxí A, Gifre L, Peris P, Parés A. A randomized trial comparing monthly ibandronate and weekly alendronate for osteoporosis in patients with primary biliary cirrhosis. Hepatology 2013 58: 2070-8. [PubMed: 23686738](Controlled trial comparing two year course of alendronate weekly vs ibandronate monthly in 33 patients with primary biliary cirrhosis, found no worsening of liver tests with either drug during therapy).
- Suresh E, Pazianas M, Abrahamsen B. Safety issues with bisphosphonate therapy for osteoporosis. Rheumatology (Oxford) 2014; 53: 19-31. [PubMed: 23838024](Systematic review of the adverse events associated with long term bisphosphonate therapy focusing upon osteonecrosis, atrial fibrillation, cancer and atypical femoral fractures, and mentioning that isolated cases of liver injury have been reported with alendronate [Yanik, 2007] and zolendronate [Polyzos, 2011]).
- Tadrous M, Wong L, Mamdani MM, Juurlink DN, Krahn MD, Lévesque LE, Cadarette SM. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos Int 2014; 25: 1225-35. [PubMed: 24287510](Systematic review of 50 publications mentioning the gastrointestinal adverse events associated with long term bisphosphonate therapy; does not mention liver toxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 published reports of drug induced liver injury between 1996 and 2012, none were attributed to a drug for osteoporosis).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, only 1 case was attributed to a bisphosphate, a self limited case of acute hepatitis with jaundice that was considered "possibly" due to zoledronic acid).
- Jiang Y, Fu Y, Xing XP, Li M, Wang O, Xia WB, Meng XW. Zoledronic acid-induced hepatotoxicity relieved after subsequent infusions in a Chinese woman with glucocorticoid-induced osteoporosis. Eur J Med Res 2015; 20: 68. [PMC free article: PMC4546306] [PubMed: 26297149](52 year old Chinese woman with corticosteroid induced osteoporosis developed fever after a first infusion of zoldedronate and had transient serum enzyme elevations [biliurbin 0.5 mg/dL, ALT 254 U/L, Alk P 118 U/L], resolving within 1 week and not recurring with subsequent yearly infusions).
- Lu Y, Pei Y, Shao Y, Yan S, Ma L, Fang F, Jin M, et al. Hepatotoxicity induced by zoledronic acid in an aged woman with primary osteoporosis. EXCLI J 2013; 12: 115-7. [PMC free article: PMC4803018] [PubMed: 27034632](73 year old woman developed flu like symptoms and had abnormal serum enzymes 3 days after an initial infusion of zoledronic acid [bilirubin not provided, ALT rising from 17 to 169 U/L, GGT from 41 to 85 U/L], falling into the normal range within 2 weeks).
- Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol 2015; 11: 418-28. [PubMed: 25963272](Review of osteoporosis, its management and treatment, mentions that intravenous bisphonosphates produce an acute phase response in one-third of patients).
- Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res 2015; 27: 413-8. [PubMed: 25534961](Among 626 postmenopausal women with osteoporosis, serum Alk P levels were elevated in 14%, largely due to bone rather than hepatic sources; treatment with alendronate and risedronate led to decreases in levels into the normal range in 65 of 84 subjects).
- Rossini M, Adami G, Adami S, Viapiana O, Gatti D. Safety issues and adverse reactions with osteoporosis management. Expert Opin Drug Saf 2016; 15: 321-32. [PubMed: 26699669](Review of the safety of drugs for osteoporosis mentions that intravenous nitrogen-containing bisphosphonates cause an acute phase response in about 30% of patients, marked by transient flu-like symptoms within a day of administration that resolves within 3 days and decreases in severity with subsequent infusions).
- Schneider JS, Montani M, Stickel F. Drug-Induced Autoimmune Hepatitis following Treatment with Zoledronic Acid. Case Rep Gastroenterol 2017; 11: 440-5. [PMC free article: PMC5624238] [PubMed: 29033761](73 year old woman developed fever, fatigue and pruritus and elevations in serum enzymes shortly after an initial infusion of zoledronic acid for osteoporosis [bilirubin not provided, ALT 183 U/L, GGT 639 U/L], ultimately treated with prednisone after enzymes remained elevated for 4 weeks, with an initial response and relapse when withdrawn 10 weeks later requiring reinitiation of immunosuppressive therapy).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Bisphosphonates: from the laboratory to the clinic and back again.[Bone. 1999]Review Bisphosphonates: from the laboratory to the clinic and back again.Russell RG, Rogers MJ. Bone. 1999 Jul; 25(1):97-106.
- Review New bisphosphonates in the treatment of bone diseases.[Drugs Aging. 1999]Review New bisphosphonates in the treatment of bone diseases.Gatti D, Adami S. Drugs Aging. 1999 Oct; 15(4):285-96.
- Review Bisphosphonates for prevention of postmenopausal osteoporosis.[Dan Med Bull. 2002]Review Bisphosphonates for prevention of postmenopausal osteoporosis.Ravn P. Dan Med Bull. 2002 Feb; 49(1):1-18.
- Management of postmenopausal osteoporosis and the prevention of fractures.[Panminerva Med. 2014]Management of postmenopausal osteoporosis and the prevention of fractures.Gambacciani M, Levancini M. Panminerva Med. 2014 Jun; 56(2):115-31. Epub 2014 Jun 19.
- Review Bisphosphonates: the first 40 years.[Bone. 2011]Review Bisphosphonates: the first 40 years.Russell RG. Bone. 2011 Jul; 49(1):2-19. Epub 2011 May 1.
- Bisphosphonates - LiverToxBisphosphonates - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...