NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Anagrelide is an antithrombotic and platelet reducing agent that is used to treat the thrombocythemia associated with myeloproliferative diseases. Anagrelide has had limited clinical use, but has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury.
Background
Anagrelide (an ag' re lide) is platelet reducing agent that is used to treat thrombocytosis due to myeloproliferative diseases. Its mechanism of action is not well defined, but it appears to inhibit the maturation and differentiation of megakaryocytes, and both synthesis and release of platelets as well as subsequent platelet aggregation. In addition, anagrelide inhibits phosphodiesterase-3 which causes vasodilation and may account for many of its side effects. In several open label trials, anagrelide was shown to reduce platelet counts in patients with thrombocythemia due to essential thrombocytosis and other myeloproliferative diseases. Severe thrombocythemia is associated with an increased risk of arterial and venous thromboses including transient ischemic attacks, stroke, myocardial infarction and other thrombotic ischemic conditions. Less commonly, thrombocytosis is associated with venous thromboses including deep vein thrombosis, pulmonary embolus and portal or splanchnic vein thrombosis. Anagrelide was approved for use in thrombocythemia due to myeloproliferative diseases in 1997, but is considered a second line agent, appearing to be less effective and less well tolerated than hydroxyurea. Anagrelide is available in capsules of 0.5 mg generically and under the brand name Agrylin. The typical initial dose is 0.5 mg daily, with subsequent gradual and monitored dose escalation based upon platelet counts and tolerance, not to exceed 10 mg daily or 2.5 mg in a single dose. It is often given in combination with aspirin. Side effects can include headache, dizziness, palpitations, fatigue, nausea, abdominal pain, dyspnea, cough, fever, edema, rash, chest pain and tachycardia. Rare, but potentially severe adverse reactions include arrhythmias, prolongation of the QTc interval, excessive bleeding and interstitial nephritis.
Hepatotoxicity
In preregistration studies, anagrelide was not associated with serum enzyme elevations or with episodes of clinically apparent liver injury. Since its approval, there has been a single published abstract reporting progressive, ultimately fatal cholestasis after liver transplantation and use of anagrelide, but there have been no other published reports of anagrelide hepatotoxicity in the literature. In large, long term follow up studies there have been occasional instances of transient serum enzyme elevations without jaundice or symptoms. The product label for anagrelide mentions abnormal enzymes as an adverse event but not clinically apparent liver injury, hepatitis or jaundice. However, the general clinical experience with anagrelide has been limited.
Likelihood score: E* (unlikely, but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which anagrelide might cause serum aminotransferase elevations or liver injury is not known. The typical daily dose is low (1 to 10 mg), which may account for its relative lack of hepatotoxicity.
Drug Class: Antithrombotic Agents
Other Drugs in the Subclass (Primary Thrombocythemia): Hydroxyurea, Interferon alfa, Aspirin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Anagrelide – Generic, Agrylin®
DRUG CLASS
Antithrombotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
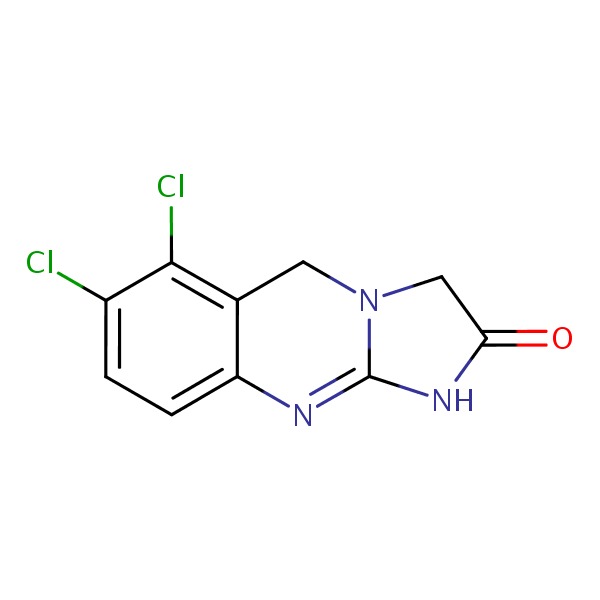
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Anagrelide | 68475-42-3 | C10-H7-Cl2-N3-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 July 2017
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman, HJ. Hepatotoxicity. The adverse effects of drugs and other chemicals upon the liver. 2nd edition. Philadelphia. Lippincott, 1999. pp 673-708.(Textbook of drug induced liver injury published in 1999; does not discuss anagrelide).
- Silverstein MN, Petitt RM, Solberg LA Jr, Fleming JS, Knight RC, Schacter LP. Anagrelide: a new drug for treating thrombocytosis. N Engl J Med 1988; 318: 1292-4. [PubMed: 3362187](Among 20 patients with thrombocytosis [>800,000] of various causes who were treated with anagrelide [1.5-4.0 mg daily] for up to 28 months, platelet counts decreased in 18 patients and side effects were mild: "except for the decrease in the platelet count, all other laboratory measurements remained normal throughout the study").
- Anagrelide, a therapy for thrombocythemic states: experience in 577 patients. Anagrelide Study Group. Am J Med 1992; 92: 69-76. [PubMed: 1731512](Among 577 patients with thrombocythemia treated with anagrelide for up to 5 years, side effects included hypotension, peripheral edema, palpitations, headache, dizziness, nausea, bloating, diarrhea, severe pancreatitis and pulmonary fibrosis; "one patient developed abnormalities in hepatocellular enzymes that resolved when the drug was discontinued and recurred on re-challenge").
- Anagrelide for essential thrombocythemia. Med Lett Drugs Ther 1997; 39 (1016): 120. [PubMed: 9422046](Concise review of the pharmacology, clinical efficacy, safety and costs of anagrelide shortly after its approval for thrombocytosis in the US; does not mention serum enzyme elevations or hepatotoxicity).
- Lin S, Howell DN, Tuttle-Newhall JE, Heneghan MA. Cholestatic liver failure secondary to anagrelide. Am J Gastroenterol 2003; 98: S153. [Abstract, Not in PubMed](48 year old man with a liver transplant for sclerosing cholangitis developed progressive cholestasis "shortly after" starting anagrelide and later died; no details provided).
- Birgegård G, Björkholm M, Kutti J, Lärfars G, Löfvenberg E, Markevärn B, Merup M, et al. Adverse effects and benefits of two years of anagrelide treatment for thrombocythemia in chronic myeloproliferative disorders. Haematologica 2004; 89: 520-7. [PubMed: 15136214](Among 60 patients with thrombocythemia due to myeloproliferative diseases treated with anagrelide [0.5 to 5 mg daily] for 2 years, response rates were 67%, and side effects included palpitations [70%], headache [52%], nausea [35%], diarrhea [33%], edema [22%] and fatigue [23%]; no mention of ALT elevations and no reported hepatic severe adverse events).
- Cacciola RR, Cipolla A, Di Francesco E, Giustolisi R, Cacciola E. Treatment of symptomatic patients with essential thrombocythemia: effectiveness of anagrelide. Am J Hematol 2005; 80: 81-3. [PubMed: 16138352](Analysis of platelet markers and coagulant activity in 17 patients with thrombocythemia treated with anagrelide [0.5 to 6 mg daily] showed resolution of symptoms of erythromelalgia [painful burning and erythematous extremities] in 9 patients and decrease in platelet aggregation and coagulant activity; no mention of ALT elevations or hepatotoxicity).
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, et al.; United Kingdom Medical Research Council Primary Thrombocythemia 1 Study. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005; 353: 33-45. [PubMed: 16000354](Among 809 patients with thrombocythemia on chronic aspirin therapy who were treated with either anagrelide or hydroxyurea for a median of 39 months, arterial thromboses and malignant transformation were less with hydroxyurea, while venous thromboses were less with anagrelide; slide effects of anagrelide included palpitations, abdominal pain and diarrhea and 4 patients had "abnormal liver function tests" compared to none on hydroxyurea).
- Fruchtman SM, Petitt RM, Gilbert HS, Fiddler G, Lyne A; Anagrelide Study Group. Anagrelide: analysis of long-term efficacy, safety and leukemogenic potential in myeloproliferative disorders. Leuk Res 2005; 29: 481-91. [PubMed: 15755500](Long term follow up on a cohort of 3660 patients with thrombocytosis who were treated with anagrelide for up to 7 years found no evidence of increased risk of leukemia; no mention of clinically apparent liver injury or deaths from liver disease).
- Emadi A, Spivak JL. Anagrelide: 20 years later. Expert Rev Anticancer Ther 2009; 9: 37-50. [PubMed: 19105705](Extensive review of the causes, clinical course and complications of thrombocythemia and role of anagrelide which is most effective in preventing venous thromboses; mentions serious cardiac, pulmonary and renal side effects, but not ALT elevations or clinically apparent hepatotoxicity).
- Gugliotta L, Besses C, Griesshammer M, Harrison C, Kiladjian JJ, Coll R, Smith J, et al. Combination therapy of hydroxycarbamide with anagrelide in patients with essential thrombocythemia in the evaluation of Xagrid(R) efficacy and long-term safety study. Haematologica 2014; 99: 679-87. [PMC free article: PMC3971078] [PubMed: 24334294](Analysis of a large prospectively followed cohort of patients with thrombocythemia focusing upon 307 of the 3643 who were receiving the combination of anagrelide and hydroxycarbamide with discussion of complications of major complications, but does not mention liver related severe adverse events).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury seen over a ten year period at 8 US medical centers, none were attributed to anagrelide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Pharmacokinetics, bioequivalence, tolerability, and effects on platelet counts of two formulations of anagrelide in healthy volunteers and patients with thrombocythemia associated with chronic myeloproliferation.[Clin Ther. 2009]Pharmacokinetics, bioequivalence, tolerability, and effects on platelet counts of two formulations of anagrelide in healthy volunteers and patients with thrombocythemia associated with chronic myeloproliferation.Petrides PE, Gisslinger H, Steurer M, Linkesch W, Krumpl G, Schüller A, Widmann R. Clin Ther. 2009 Feb; 31(2):386-98.
- Adverse effects and benefits of two years of anagrelide treatment for thrombocythemia in chronic myeloproliferative disorders.[Haematologica. 2004]Adverse effects and benefits of two years of anagrelide treatment for thrombocythemia in chronic myeloproliferative disorders.Birgegård G, Björkholm M, Kutti J, Lärfars G, Löfvenberg E, Markevärn B, Merup M, Palmblad J, Mauritzson N, Westin J, et al. Haematologica. 2004 May; 89(5):520-7.
- Review Anagrelide-associated Cardiomyopathy and Heart Failure in a Patient with Essential Thrombocythemia: A Case Report and Literature Review.[Intern Med. 2022]Review Anagrelide-associated Cardiomyopathy and Heart Failure in a Patient with Essential Thrombocythemia: A Case Report and Literature Review.Sugawara M, Okada S, Kanda M, Iseki T, Sakaida E, Kobayashi Y. Intern Med. 2022 Nov 1; 61(21):3293-3299. Epub 2022 Mar 26.
- TPO, but not soluble-IL-6 receptor, levels increase after anagrelide treatment of thrombocythemia in chronic myeloproliferative disorders.[Int J Med Sci. 2008]TPO, but not soluble-IL-6 receptor, levels increase after anagrelide treatment of thrombocythemia in chronic myeloproliferative disorders.Palmblad J, Björkholm M, Kutti J, Lärfars G, Löfvenberg E, Markevärn B, Merup M, Mauritzson N, Westin J, Samuelsson J, et al. Int J Med Sci. 2008 Apr 13; 5(2):87-91. Epub 2008 Apr 13.
- Review Anagrelide, a selective thrombocytopenic agent.[Am J Health Syst Pharm. 1998]Review Anagrelide, a selective thrombocytopenic agent.Oertel MD. Am J Health Syst Pharm. 1998 Oct 1; 55(19):1979-86.
- Anagrelide - LiverToxAnagrelide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...