This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Louisiana currently has one of the highest rates of obesity in the United States. Obesity accounts for $2.4 billion in yearly health care expenditures in Louisiana, 42.5% of which is financed by the Centers for Medicare & Medicaid Services (CMS). Obesity disproportionately affects underserved populations, and Louisiana is characterized by high levels of poverty, low health literacy, and food insecurity. The US Preventive Services Task Force recommended that physicians offer intensive multicomponent behavioral interventions to individuals with obesity, and CMS covers intensive behavioral therapy for obesity delivered by a qualified primary care provider (PCP). However, the reliance on PCPs to deliver intensive behavioral therapy for obesity has limitations, creating a critical need to test models that incorporate the 2013 American Heart Association/American College of Cardiology/The Obesity Society guidelines for the management of overweight and obesity in adults in real-life settings for obesity treatment in primary care clinics.
Objectives:
The primary aim of this trial was to develop and test the effectiveness of a 24-month, patient-centered, pragmatic, and scalable obesity treatment program delivered within primary care in an underserved population. We hypothesized that relative to patients who received usual care (UC) from their PCP, patients who received an intensive lifestyle intervention (ILI) delivered by health coaches embedded in a primary care setting would have (1) greater percentage reductions in body weight and (2) significant improvements in quality of life (QOL), functional capacity, medical care satisfaction, and obesity comorbidities. Additionally, 3 secondary aims (1) evaluated the relationships between adherence to intervention components and corresponding changes in body weight and secondary outcomes; (2) examined the effects of the intervention on system-level practices and patient satisfaction with care; and (3) tested the heterogeneity of treatment effects (HTE) across clinics and subgroups of patients by sex, age, and race.
Methods:
We conducted a cluster randomized controlled trial in which 18 Louisiana primary care clinics were randomized equally to either the ILI or UC group. A total of 803 (452 UC, 351 ILI) adults (67% African American; 65.5% with annual household income of <$40 000) with obesity were enrolled. The UC group received their normal care from their primary care team. The ILI group received a program consisting of weekly sessions in the first 6 months (which represented an active weight loss period), followed by monthly sessions for the remaining 18 months (which represented a weight loss maintenance period), all delivered by health coaches embedded in the clinics. The multicomponent ILI included the provision of portion-controlled foods for the first 4 weeks, shared goal setting for diet and physical activity, and daily weighing on an electric scale with weight loss feedback to the coaches and patients through a computer tracking system. A “toolbox” approach provided specific nutritional, physical activity, and behavioral strategies to choose from depending on the needs of each patient.
The primary outcome was percentage weight loss at 24 months. Secondary outcomes included changes in cardiometabolic disease risk factors, QOL, functional capacity, medical care satisfaction, obesity comorbidities, and HTE across age, sex, and race subgroups. Changes in outcome measurements from baseline to 24 months were analyzed in the context of repeated-measures linear mixed-effects models, which included random cluster (clinic) effects.
Results:
Eighteen clinics (9 ILI, 9 UC) enrolled a median of 40.5 (range, 2-89) patients per clinic. Of the enrolled patients, 83.4% completed the 24-month trial. Percentage weight loss at 24 months was significantly greater in the ILI group (−4.99% [95% CI, −6.02% to −3.96%]) than in the UC group (−0.48% [95% CI, −1.57% to 0.61%]), with a mean difference of −4.51% (95% CI, −5.92% to −3.10%) between the groups (P < .0001). Compared with the UC group, significant improvements at 24 months were found in the ILI group for total and high-density lipoprotein cholesterol but not for other cardiometabolic risk factors. Weight-related QOL at 24 months was significantly greater in the ILI group than in the UC group for numerous subscales. Patient satisfaction with medical care did not significantly change within either group.
Conclusions:
A high-intensity lifestyle–based obesity treatment program delivered in an underserved primary care population produced clinically significant weight loss and improvements in cardiometabolic risk factors and weight-related QOL over 24 months.
Limitations:
Recruitment was lower than originally anticipated (sample size estimates ensured adequate power for all analyses). The sample was composed primarily of women (84.4%), which limited our ability to examine differences in weight loss across sex-by-race subgroups.
Background
Obesity is a highly prevalent and serious medical condition in the United States, and Louisiana ranks highest among the states in the prevalence of obesity.1 Obesity increases the risk of type 2 diabetes, heart disease, poor quality of life (QOL), and several cancers.2 Indeed, Louisiana sits firmly in the “chronic disease belt,” characterized by a high prevalence of cancer,3 cardiovascular disease,4,5 diabetes,6 and obesity.7 Louisiana's population is uniquely vulnerable, as 32% of the population is African American,8 19% of residents live in poverty,8 and Louisiana is ranked 49th among the states for low levels of health literacy.9 Given that African Americans and other underserved populations have a disproportionately high obesity prevalence,10,11 identifying strategies to reduce obesity in these populations is imperative for achieving national public health goals to reduce health inequities.12
While the high obesity rates are concerning, it is equally concerning that health care systems have not delivered medical interventions capable of producing even modest weight loss.13 With primary care providers (PCPs) being the cornerstone of medical care in the United States, the US Preventive Services Task Force (USPSTF) recommends that physicians offer intensive multicomponent behavioral interventions to individuals with obesity.14 Further, the Centers for Medicare & Medicaid Services (CMS) covers intensive behavioral therapy for obesity delivered by a PCP.15 However, relying on PCPs to deliver intensive behavioral therapy for obesity has limitations, in part due to time constraints during a typical primary care visit and lack of training among PCPs in nutrition education and behavioral therapy.13,16 A narrative review of obesity management in primary care indicated that obesity treatment options delivered in primary care have limited success, demonstrating only a 1- to 3-kg weight loss over 6 to 24 months.13 As most studies only employed monthly or quarterly visits of 10 to 15 minutes in duration, this low weight loss was likely due to the low intervention intensity.13 Indeed, there is evidence that higher-intensity interventions delivered by trained interventionists in primary care can produce greater weight loss.17
The 2013 American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) guidelines for the management of overweight and obesity in adults18,19 assert that an intensive comprehensive lifestyle intervention is the centerpiece to effectively promoting weight loss and improving health. The guidelines' recommendation for comprehensive lifestyle interventions was based on a systematic review of the results from 51 trials to promote weight loss and weight loss maintenance. The guidelines emphasize the gold standard of onsite, high-intensity (ie, ≥14 sessions in 6 months) comprehensive interventions delivered by a trained interventionist. Interventionists include mainly health professionals with various specialties (eg, registered dietitians, psychologists, health counselors, exercise specialists) trained to follow weight management protocols. Obesity treatment models based on the AHA/ACC/TOS guidelines that are adaptable to real-life primary care settings are needed.
The primary aim of the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial was to test the effectiveness of a pragmatic, high-intensity lifestyle–based obesity treatment program delivered within primary care clinics. PROPEL was designed to scale to large patient populations and directly address the “translation gap” between current evidence-based obesity treatment models and primary care practice to impact the way in which CMS reimburses for obesity treatment in the future. We hypothesized the following:
- Relative to patients who receive usual care (UC), patients who receive a high-intensity, health literacy-appropriate, and culturally appropriate intensive lifestyle intervention (ILI) delivered by trained health coaches embedded in a primary care setting will have greater percentage reductions in body weight; and
- relative to patients in UC, patients who receive the ILI will have significant improvements in health-related QOL (HRQOL), satisfaction with medical care, and improved obesity comorbidities.
The 3 secondary aims of the PROPEL trial were to (1) evaluate relationships between adherence to intervention components (physical activity, diet, sessions, etc) and corresponding changes in body weight and secondary outcomes; (2) examine the effects of the intervention on system-level practices and patient satisfaction with care; and (3) test the heterogeneity of treatment effects (HTE) across clinics and across subgroups of patients (men vs women, White vs African American, older vs younger adults).
PROPEL was a cluster randomized controlled trial (RCT) in which primary care practices were randomized into either an ILI or UC group, which differs from a traditional RCT in which individual patients are randomly assigned to intervention groups. The cluster randomized design helped reduce contamination or spillover effects between the 2 intervention groups. While the unit of randomization was the clinic, the main study outcomes were measured at the individual patient level.
Patient and Stakeholder Engagement
The design of PROPEL was guided by extensive stakeholder, community, and patient engagement, as explained in Katzmarzyk et al20 and below.
Stakeholder Engagement
Stakeholders (eg, leadership and clinicians of federally qualified health centers [FQHCs]) were recruited by the study co-investigators. Stakeholder engagement was initiated before the trial's inception and continued throughout the study period via email, phone, and in-person meetings. Stakeholders identified clinics to participate in the study, assisted study investigators in obtaining the proper approvals to collect data within the clinics, identified other clinic staff members to assist study staff within each clinic, provided clinic-level statistics to be included in analyses, and recruited members of our patient engagement teams among other vital roles. Additionally, stakeholders met periodically with study investigators to discuss and solve issues as they arose, such as slow recruitment or logistical challenges within the clinics (eg, lack of space for coaches or assessment technicians within the clinics).
Patient Engagement
A series of focus groups conducted early in the trial design phase captured perceptions about obesity treatment options among health care providers and patients with low income.21 These focus groups were vital to selecting the patient-reported outcomes (eg, QOL, obesity comorbidities, etc) and designing the intervention curriculum. Three patient advisory boards (PABs; located in North Louisiana [n = 8], South Louisiana [n = 8], and New Orleans [n = 18]) that met quarterly (in person or via teleconference) throughout the study period were also instrumental in trial design and conduct. PAB members were recruited with assistance from PROPEL stakeholders and co-investigators. The PAB members were representative of the anticipated study population; most members were African American, were middle aged, and had personal experience with obesity and weight loss. The PAB members also resided in similar communities as the study participants.
The PABs provided significant input on patient recruitment and retention strategies, reviewed PCP educational materials, and provided feedback on disseminating the results to the community. PAB meetings were generally informal, with a short presentation on a topic of interest during which members interacted through questions, comments, and recommendations. The initial PAB meeting included a presentation on the basics of research, which provided a foundation for future meetings. In addition to choosing the study name and acronym, PAB members provided feedback on the study protocol and conduct, including the intervention curriculum (examples of modifications made based on PAB feedback include making edits to the literacy level, as well as adding content about eating at social events/family functions, stress, and QOL), recruitment methods (editing language on recruitment flyers to better reflect patients' interests), UC newsletter (providing community health events to include), and patient retention (helping choose visit attendance incentives, emphasizing the altruistic aspect of research participation). As the study neared completion, the PAB meetings included presentations and discussions of basic research statistics so that PAB members were better able to both understand and comment on the study's results. PAB members provided feedback and context for preliminary study results, which were presented during the final PAB meeting members will continue to be an important avenue for disseminating the study's results to the community.
Finally, 2 patient representatives met with study investigators and staff during monthly study project management committee meetings to provide feedback and oversight on the study's conduct, contribute to and approve study materials, and help troubleshoot issues with recruitment, retention, and intervention delivery. Although the project management committee patient representatives were less representative of the study's patient population than were the PAB members, their experiences with weight management as well as their work in the community enabled them to provide invaluable feedback for the study's conduct. For example, these members provided topics, content, authorship, and edits to the UC newsletters, contributed to results dissemination through edits to conference abstracts, solved logistical challenges during the study's initial protocol development and trial initiation, and represented the trial at annual conference meetings.
Community Member Engagement
A community monitoring board (CMB), composed of 6 individuals representing community organizations (university agricultural center, health education center, community health organizations), met in person yearly to provide feedback on the trial's overall direction and helped disseminate the results to the community through their networks. CMB members were recruited through participating clinics, stakeholders, and study investigators. To solicit their feedback, during the annual meetings, we gave CMB members a status update on the trial's progress and an overview of the current issues the trial was experiencing. CMB members helped identify PAB members, suggested new retention techniques, and provided publication ideas and results interpretations based on their experiences (eg, an article on retention methods has been published).
Methods
Detailed methods were previously published by our group, and the information in this section is derived from these publications.20-22
Study Overview and Design
PROPEL was a 2-group (ILI or UC) cluster RCT in which the clusters were primary care clinics in 3 geographic areas of Louisiana (New Orleans, North LA, and South LA). A total of 18 Louisiana primary care clinics inclusive of low-income populations with a high percentage of African Americans were randomized to either the (1) ILI or (2) UC group. Outcomes were assessed at baseline and 6-, 12-, 18-, and 24-month visits. The primary aim was to develop and test the effectiveness of a 24-month obesity treatment intervention delivered within primary care. The intervention was developed, in part, using information gathered from patients and health care providers through a series of focus groups21 (detailed methods are described at the end of this section). We hypothesized that relative to patients who received UC, patients who received a health literacy– and culturally appropriate ILI delivered by trained health coaches embedded in a primary care setting would have (1) greater percentage reductions in body weight and (2) significant improvements in HRQOL, satisfaction with medical care, and obesity comorbidities. The 3 secondary aims were to (1) evaluate relationships between adherence to intervention components and corresponding changes in body weight and secondary outcomes, (2) examine the intervention's effects on system-level practices and patient satisfaction with care, and (3) test HTE across clinics and across patient subgroups.
Study Setting
Primary care clinics were chosen as the study's setting in accordance with the USPSTF's recommendation that physicians offer behavioral treatment for obesity and to address the translation gap between treatment recommendations and implementation in primary care. Eighteen primary care clinics were randomized by the study statistician to either the ILI or UC group after stratification by health system. The 18 clinics were from 5 health systems (4 FQHC systems [14 clinics] and 1 large, nonprofit academic health care delivery system [4 clinics]). Randomization to the ILI or UC group was stratified by health system to ensure adequate staffing of ILI clinics and representation from all regions of the state and types of health systems in both groups of the trial. The study statistician revealed the clinics' randomization group to investigators once completed so that the ILI clinics could be properly staffed with health coaches and supplied with ILI materials. The health coaches were hired, trained, and paid by the Pennington Biomedical Research Center, the primary academic center that conducted the study. Given that this trial was randomized at the clinic level and the interventions were very distinct, as the ILI included a health coach, PROPEL patients knew their group assignment, as did clinic and study staff. However, the patients did not know any details of the other group assignment, and every effort was made to blind the study staff responsible for patient assessments (eg, assessment technicians) to patients' intervention status; all health coaches were blinded to the patients' official study measurements.
According to Federal Office of Rural Health Policy classification,23 14 of the participating clinics were in urban areas, while 4 were in rural areas. The size of the clinics (patients served) during study recruitment ranged from 1100 to 35 000.
Participants
Following the clinics' randomization, patients were recruited within the 18 primary care clinics using a variety of approaches, including (1) interactions with their PCPs, (2) searches of electronic medical records (EMRs), (3) responses to emails sent through their clinic's patient portal, (4) responses to flyers in clinic waiting areas, and (5) interactions with PROPEL staff in the clinic. Given that clinics were randomized rather than patients, all patients were recruited with specific information about the group to which their clinic was assigned; that is, patients were informed a priori about their group assignment rather than being randomized after recruitment.
A trained recruiter followed up with each patient to assess their interest in participating in the study. During this prescreening, eligibility was assessed based on self-reported responses to questions related to the inclusion/exclusion criteria provided in Table 1. An in-person screening visit was scheduled if the patient was eligible based on the prescreening criteria. Study staff read and reviewed the consent documents with all patients, and written informed consent was obtained before participation in the screening visit, which was conducted by study staff at the patient's primary care clinic. At this screening visit, a patient's height and weight were measured, and patients answered several questionnaires to determine their eligibility. The body mass index (BMI) measured at the screening visit was used to determine eligibility, which may have differed from the baseline BMI used for analysis; however, the mean BMI at the screening visit (37.4) did not differ significantly from the mean baseline BMI (37.2; P = .575).
Table 1
Inclusion and Exclusion Criteria for the PROPEL Trial.
Following the screening visit, eligible patients' PCPs reviewed their medical records alongside the PROPEL inclusion and exclusion criteria and provided medical clearance if there were no contraindications to participation. Eligible patients with medical clearance were enrolled in the trial once they completed their baseline visit, which was conducted by study staff in the primary care clinic. Patients received the intervention (ILI or UC) to which their clinic was assigned as outlined in the recruitment materials and consent forms, and patients were only told details about the intervention to which they were assigned. Intervention group assignment was not blinded because researchers and study staff needed to logistically prepare for implementation in ILI clinics differently than in UC clinics. In addition, there were various measures taken to ensure intervention fidelity, defined as the extent to which the ILI was delivered according to the protocol, including monitoring health coaches in session, which further unblinded investigators. The assessors were unblinded to group assignment simply due to the health coach's presence in certain clinics but not in others. However, patients did not know details of the other intervention assignment (separate consent forms were used for the UC and ILI groups), assessment technicians were blinded to the details of patients' intervention participation during data collection, and both investigators and health coaches were blinded to the assessment data until all data collection was complete. The Pennington Biomedical Research Center IRB reviewed and approved the study protocol and all research materials.
Interventions and Comparators
ILI Group
Trained health coaches, who were employed by Pennington Biomedical Research Center and embedded in the primary care clinics, delivered a comprehensive, high-intensity intervention program (ILI) as recommended by the 2013 AHA/ACC/TOS obesity guidelines.19 This intervention program was designed based on the Diabetes Prevention Program (DPP),24 Look AHEAD (Action for Health in Diabetes),25 and Comprehensive Assessment of the Long-term Effects of Reducing Energy Intake (CALERIE)26 studies. However, given the prevalence of low health literacy in Louisiana,9 all intervention materials and approaches were adapted to be health literacy appropriate through extensive consultation with PABs and health literacy experts (T.C.D. and C.L.A.). Health coaches were trained in principles of health literacy and motivational interviewing techniques to enhance patient communication and encourage patient activation.27,28 Health coaches had earned at least a bachelor's degree in nutrition, physical activity, or behavioral medicine. Of the 8 health coaches, 6 had master's degrees in nutrition, dietetics, exercise physiology, or counseling, and 5 were registered dietitian nutritionists. Fifty percent of the health coaches (n = 4) were African American and 50% were White, and all health coaches resided in the Louisiana community in which they coached.
Enrolled patients in ILI clinics attended weekly sessions in the first 6 months; 16 of these were completed in person in individual or small-group sessions and 6 via phone sessions (1 phone session per month). Sessions then occurred at least monthly and alternated between in-person (individual or small groups of 2-3 patients) and phone sessions for the remaining 18 months, though increased contact with the health coach via in-person or phone sessions was an option that was used if patients were struggling to meet their goals. In addition to the session attendance frequency defined by the protocol (weekly for 6 months, monthly for 18 months), patients met with their coach as often as they wished, assuming that the coach's schedule could accommodate it and there were no contraindications identified by the intervention team. There were 43 intervention session topics, which included the use of portion-controlled foods and meal replacements, increasing physical activity, calorie balancing, self-monitoring, and dealing with stress, among other topics. The session topics were delivered in order as shown in Appendix A, Supplementary Table S1; however, coaches were encouraged to review additional session materials on topics with which the patient needed more support (ie, upcoming family functions with food often necessitated the “Stay on track at family events” session material; healthy snacking, fast food, and dealing with stress were other sessions often reviewed multiple times with patients). Coaches tracked additional contacts that were in excess of the 43 scheduled intervention sessions. Additional contacts occurred via various methods, such as additional in-person meetings, simple text messages reminding the patient to weigh themselves, a phone conversation to help the patient choose a healthy lunch option, etc.
Given that greater initial weight loss is associated with long-term weight loss and weight loss maintenance,29,30 a strong focus of the intervention was on maximizing initial (3-month) weight loss. The meal plan for weeks 1 to 4 included portion-controlled foods, including easily available and affordable items. Prepackaged portion-controlled foods and meal-replacement shakes were provided to all ILI participants free of charge as a component of the ILI during weeks 1 to 4 and were available as part of our “toolbox” approach after week 5. Meal plans became less structured over time as participants learned to manage portion size and energy intake. Prepackaged foods were distributed as a toolbox option if it was clear that the participant might benefit and if attempts to secure similar foods failed. These foods were provided on a time-limited scale, usually 1 to 2 weeks, while solutions to the participant's food access problem were identified. Participants were also provided measuring cups and portion-appropriate plates to help attain their dietary goals. Meal plans were consistent with dietary guidelines (eg, ∼55% carbohydrate, ∼15% protein, and ∼30% fat),31 included nutrient-dense foods (eg, whole grains, fruits, lean protein), and were formatted for easy dissemination through clinics. Given Louisiana's unique culinary environment, the ILI curriculum and coaching sessions also included ways in which to incorporate common Southern food staples (eg, red beans and rice, gumbo) in a healthier way.
An important component of the ILI was shared short-term goal setting between the health coach and participant to reach the broader study goals. A personal goal of 10% weight loss was set for participants, and they were coached to set their own goals and develop eating and physical activity action plans to meet that goal. Given the importance of physical activity for successful weight loss and weight loss maintenance, the second target for this trial was for patients to achieve at least 175 minutes per week of moderate-to-vigorous physical activity. A major focus of the physical activity intervention was on increasing lifestyle activities, such as taking the stairs instead of the elevator, parking further away, actively commuting (walking or riding a bicycle), and replacing sedentary activities with more-active options. A pedometer (New Lifestyles model SW200) was provided to all ILI participants along with directions on how to increase their daily step counts.
A novel aspect of the PROPEL intervention was the addition of a computer tracking system (CTS), which facilitated tailoring the intervention to the individual participant and promoting intervention fidelity among clinics, health coaches, and participants.26 A weight loss calculator was used to (1) calculate personalized daily energy intake targets for each participant that, if met, would result in 10% weight loss over 6 months; and (2) create a weight graph that would display each participant's predicted weight loss over time. This weight graph included a zone that reflected being adherent to the diet; plotting the participant's weight over time in relation to the zone determined if they met their energy intake target and lost weight at the rate expected (see Figure 1).32-35 The zone included a 10% weight loss target represented by a line in the middle of the zone, with lower and upper bounds that represent ∼7.5% and ∼12.5% weight loss, respectively. The upper and lower bounds of the graph accommodated biological variation and error in the prediction model. Participants were encouraged to weigh themselves daily with a study-provided electronic scale (BodyTrace). The scale uploaded data to the CTS in real time. In turn, the data were plotted onto the personal weight graph, which was available in real time to both the coach and the participant via any internet-connected device and thus provided a platform to intervene quickly (eg, between sessions) should the participant need additional support. Health coaches used the weight graphs to identify participants struggling to meet their weight loss goals (those whose weights plotted above the target zone), which triggered the health coach to deploy strategies from the toolbox.
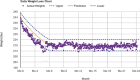
Figure 1
Example of a Personal Weight Loss Graph for a Single ILI Participant Followed for 24 Months.
In addition to the health coaching sessions, CTS, and the provision of portion-controlled foods, the ILI also included a toolbox of additional strategies. The toolbox approach was successfully deployed in previous clinical trials and led to improvements in intervention efficacy.24-26 Additionally, the toolbox enabled health coaches to tailor treatment to address patient preferences and lifestyles, as well as regional and cultural differences among patients. Supplementary Table S2 in Appendix A provides all toolbox options as well as their respective frequencies of use. This approach was highly individualized, and the health coach worked with the participant to develop acceptable strategies, including how long they would be deployed and how to evaluate their effectiveness.
As previously noted, intervention fidelity was facilitated by hiring coaches residing in the communities where the clinics are located, providing health coaches with extensive initial training and yearly updated training sessions, and using the CTS and toolbox to help standardize treatment delivery. In addition to formal monitoring sessions, the health coaches regularly audio-recorded sessions that were reviewed by the intervention team to ensure that they were delivering the intervention similarly across clinics and using the CTS and toolbox as intended. Finally, a weekly case conferencing meeting was held via webinar for coaches to discuss participants with the research team and to share feedback on intervention strategies. All health coaches were required to attend these meetings. See Appendix B for more details on the trial's intervention fidelity measures.
In addition to the patient-centered ILI program itself, PCPs of patients in the ILI group received an obesity science education program. This education included information on the management of obesity, the co-management of morbidities such as type 2 diabetes and hypertension, minimizing bias and stigma related to obesity, and principles of health literacy. The PCP education program was delivered in a series of webinars and face-to-face seminars conducted in the clinics by experts in obesity, patient engagement, and health literacy. PCPs in the ILI clinics also received access to the webinar series via a password-protected website.
UC Group
Enrolled patients in UC clinics received their UC from their primary care team throughout the 24-month period. Although patients in the UC group were not required to see their PCP for weight management counseling, they were provided a series of health-related newsletters, and their PCPs were provided with a webinar related to weight management in primary care. To maintain contact and aid in the retention of patients in the UC group, we sent them 3 newsletters per year on selected topics, along with a listing of health promotion events offered in their community. Newsletter topics included the importance of reducing sedentary behavior, sleep hygiene, brain and memory health, smoking cessation, etc. The care received by patients in the UC group was measured with a patient-provider relationship survey; this survey was completed by patients in both groups at the baseline and 24-month assessment visits.
The PCPs in UC clinics received a baseline presentation that described weight management in primary care settings and the current CMS approach to reimbursement for obesity treatment.15 Additionally, an informational brochure on the current CMS approach to reimbursement for obesity treatment was sent to the PCPs at least once per year throughout the trial. PCPs in UC clinics also had access to the baseline webinar presentation and the informational brochure throughout the study via a password-protected website.
Study Outcomes
Study outcome data were collected from patients or their PCPs by trained assessment technicians within the primary care clinics.
Primary Outcome Measure
The primary outcome measure of the PROPEL trial was the percentage change in body weight from baseline to month 24. Body weight was measured in duplicate with the patient in light clothing without shoes to the nearest 0.1 kg using a digital scale (Seca Model 876) at each assessment visit. The patient was instructed to stand still in the middle of the scale with head erect and eyes looking straight ahead.
Secondary Outcome Measures
Secondary outcome measures included absolute 24-month changes in body weight, waist circumference, blood pressure, fasting glucose and lipids, and QOL measures. Assessments were conducted by trained technicians with no involvement in intervention delivery. Table 2 provides an overview of the data measurement and collection schedule for PROPEL, as described below.
Table 2
Data Measurement and Collection Schedule for Patient Data.
Anthropometric measurements
Standing height was measured with a portable stadiometer (Seca Model 213) at the baseline visit. Patients were asked to remove their shoes and have their heels, buttocks, and upper part of the back remain in contact with the stadiometer. The patient was asked to inhale and hold their breath, while 1 technician applied traction to the patient's head to maintain alignment with the Frankfort Plane. The slide was lowered until it reached the vertex of the skull. Waist circumference was measured at all assessment visits using a nonelastic anthropometric tape (Graham-Field model 1340-2). The measurement was made by 1 technician, while a second maintained a parallel position of the tape midway between the lower rib margin and the iliac crest, at the end of gentle expiration.
Blood pressure and pulse measurements
Resting systolic and diastolic blood pressures were measured using a validated automated Omron device (HEM-907XL digital blood pressure monitor) at baseline and all follow-up visits. After the patient sat quietly alone for at least 5 minutes, 2 sitting measurements were made 1 minute apart.
Fasting glucose and lipids
Finger-stick blood samples were obtained to measure fasting levels of lipids (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides) and glucose using a validated Alere Cholestech LDX Analyzer at the baseline, 12-month, and 24-month visits. The patients were asked to fast for at least 10 hours before their visit. The Cholestech Analyzer was checked for accuracy using standard controls each day before it was used to analyze patient blood samples.
Concomitant medications
Patients were asked to bring their medications or a list from their pharmacist/PCP to the screening visit and all subsequent assessment visits. A concomitant medications form was completed at each visit to track changes in the patient's medications and dosages throughout the trial.
Adverse events
An adverse event (AE) surveillance form was used to screen for AEs and serious AEs (SAEs) at each assessment visit. All SAEs were recorded, and those that were unexpected and related or possibly related to the study were reported to the IRB in accordance with their requirements.
Health literacy
The Rapid Estimate of Adult Literacy in Medicine–Short Form (REALM-SF) is a 7-item health literacy assessment that was administered at the screening visit.36 The patient was asked to read health-related words aloud. Two words (flu and pill) were not scored but were used to identify patients who were ineligible due to their inability to read and comprehend the informed consent documents. The score ranges from 0 to 7. Those scoring below 7 were considered to have low health literacy (reading below a ninth grade level), and those scoring below 4 were considered to have very low health literacy (reading below a third grade level).19 Patients scoring 0 to 3 on the REALM-SF were provided assistance in completing the other questionnaires using a semistructured interview. Patients scoring 4 to 7 on the REALM-SF completed the other questionnaires on their own, but assistance was provided as requested.
Demographics and health history
A self-report demographic and health history questionnaire was administered at the screening visit, which collected information about age, sex, race/ethnicity, use of cigarettes and alcohol, health insurance status, postmenopausal status, income and employment, education level, and history of chronic diseases. Changes in health status were captured by self-report at the 6-, 12-, 18-, and 24-month assessment visits.
Food security
Household food security was measured at baseline and at the 12- and 24-month assessment visits using a 6-item subscale of the 12-month Food Security Scale Questionnaire.37 Scores range from 0 to 6, and a score of 2 or higher indicates food insecurity.
Provider demographics and knowledge (PCP survey)
Each patient's PCP was asked to complete a survey before the trial began and at the trial's end. The provider survey was adapted from the Hopkins Practice-based Opportunities for Weight Reduction (POWER) trial.38 The questionnaire assessed the PCP's demographic information and knowledge about weight management practices.
Physical activity
Physical activity levels were self-reported at baseline and at the 6-, 12-, and 24-month assessment visits using the International Physical Activity Questionnaire–Short Form (IPAQ-SF),39 which asks questions related to physical activity performed over the previous 7 days. The IPAQ-SF has acceptable reliability39 and is sensitive to change within the context of a weight loss intervention.40
Dietary intake
A dietary fat, fruit, vegetable and alcohol intake questionnaire was administered at baseline and at the 6-, 12-, and 24-month assessment visits. The questionnaire contained scales derived from several sources: the National Cancer Institute (NCI) fat screener,41 the NCI and National 5 a Day Program fruit and vegetable screener,42,43 and the Brief Questionnaire to Assess Habitual Beverage Intake (BEVQ-15).44 The questionnaire asked patients to report the frequency of consuming specific foods and beverages over the past 12 months. Two scores were created in addition to the percentage energy from fat: number of times per day a fruit or vegetable (fruit, fruit juices, vegetables, salad, and potatoes) was consumed and number of times per day any type of alcohol was consumed.
Eating attitudes and behaviors
The Three-Factor Eating Inventory measures dimensions of eating attitudes and behaviors. Three factor-analyzed subscales (cognitive restraint, disinhibition, and hunger) are derived from the questionnaire.45 For the purpose of the PROPEL trial, only the cognitive restraint and disinhibition subscales were administered to patients. This questionnaire was administered at baseline and at the 6-, 12-, and 24-month assessment visits.
Patient-provider relationships
A patient-provider relationship survey was completed by each patient at the baseline visit and at the 24-month assessment visit. This 11-item instrument was adapted from the POWER trial.38 POWER investigators compiled the patient-provider survey from several sources, including the validated Consumer Assessment of Healthcare Providers and Systems (CAHPS) 2.0 Adult Core Survey.46 A CAHPS global rating of the provider (rating from 0-10) was also included. Finally, a question was included to address patient centeredness or the perception of “being known as a person.”47 The framing of all questions in the survey was adapted by asking patients about their care over the past 2 years given that both the POWER and PROPEL trials were 2 years in duration. A summary patient-provider relationship score (8-32) was computed by adding the total responses from the 8 questions (after reversing the 2 opposite scales).
Intervention satisfaction
A self-report questionnaire that asked about personal experience and satisfaction with the intervention was administered to patients enrolled in the ILI group at the 6- and 24-month assessment visits. This questionnaire was developed by the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) study48 and included questions obtained from the Veterans Affairs Diabetes Prevention Program (VA DPP), Rural Lifestyle Eating and Activity Program (Rural LEAP), and POWER trials. It asked questions about the convenience of the clinic's location and appointment times, feelings about the health coach, and the perceived effectiveness and organization of the intervention curriculum and delivery.
Generic HRQOL
Generic HRQOL was measured using the 29-item Patient-Reported Outcomes Measurement Information System (PROMIS-29) instrument.49,50 The questionnaire includes health-related domains of physical function/functional capacity, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles and activities, pain interference, and pain intensity. PROMIS surveys were designed and tested to be easy to understand and navigate. The PROMIS-29 was administered at the baseline visit and at the 6-, 12-, and 24-month visits.
Weight-related QOL
The Impact of Weight on Quality of Life–Lite (IWQOL-Lite) is a 31-item questionnaire designed to measure obesity-specific aspects of QOL, which produces a total score and separate scores for physical function/functional capacity, self-esteem, sexual life, public distress, and work or daily activities.51,52 The IWQOL-Lite was administered at baseline and at the 6-, 12-, and 24-month assessment visits.
Assessment Data Monitoring and Quality Assurance
The PROPEL trial implemented a data monitoring plan to ensure that data in the electronic data capture system accurately reflected the patients' responses, which confers greater confidence to the conclusions of the trial's effectiveness. Assessment technicians were trained on the study protocol and assessment procedures in an initial training session upon hire and annually at refresher training sessions. The assessment technicians' adherence to the study protocol and source document verification, verifying that the paper data collection form completed by the patient matched what data were entered, was monitored by study management during visits to each clinic 1 to 2 times per year. Additionally, study staff performed source document verification on 100% of the assessment data. Data entry errors and inconsistencies were corrected upon detection. Finally, the data manager and trial biostatistician conducted thorough data cleaning to identify any remaining inconsistencies once all data were collected. See Appendix C for more information on the PROPEL Data Management Plan.
Sample Size Calculations and Power
As 3% to 5% weight loss is likely to result in clinically meaningful health outcomes for adults who are overweight or have obesity,19 the sample size calculations for the primary outcome were powered to detect differences in mean percentage of baseline weight loss in the ILI group of 3.5% at 24 months, relative to UC. Power calculations used a nominal α = .05 significance level with a 2-tailed test, with minimal power of 80% for the planned subgroup analyses (men vs women; White vs African American; older vs younger adults). Based on the Louisiana Obese Subjects Study (LOSS) study,53 the SD for percentage weight change for patients nested within clinics was 8.4%. For the subgroup analyses, a net sample size of 184 patients (92/group) was sufficient for detecting a 3.5% weight loss with 80% power when randomization was performed at the patient level. After accounting for the design effect (estimated to be 1.8 from the LOSS study53), at least 134 patients per group were required to realize 80% power in a trial randomized at the clinic level. Allowing for 30% attrition, at least 192 patients per group were needed, with a total (2 groups) enrollment of at least 384 patients per subgroup analysis; this translated into a total sample size of at least 768 patients, providing at least 97% power to detect a mean weight loss in the ILI group of 3.5% at 24 months, relative to UC in the total sample.
Time Frame for the Study
The study was organized into 3 periods: (1) study planning, (2) data collection, and (3) analysis. During the study planning period, which occurred from January 2015 to April 2016, the intervention and assessment materials were adapted, focus groups were conducted, IRB approvals were obtained, and study staff were hired. Data were collected from April 2016 through September 2019, during which time patients and PCPs received the intervention or educational program to which their clinic was assigned (Figure 2). Data analysis began in September 2019 when the final patient completed their 24-month assessment visit and continued through the end of the grant period in December 2020. Additionally, data analysis of secondary manuscripts will continue beyond 2020.
Figure 2
Overview of the PROPEL Data Collection Period.
The 18 participating clinics began recruitment in 2 phases. Phase 1 recruitment (10 clinics) began in April 2016, and phase 2 recruitment (8 clinics) began in October 2016. Patients were recruited between April 2016 and September 2017 and participated in either the UC or ILI group for 24 months. The intervention length was chosen to examine both an active weight loss period hypothesized to occur mostly in the first 6 months and a weight maintenance period during the final 18 months. Assessment time points were selected to capture initial weight loss (eg, at 6 months) and whether effects were sustained over time (eg, at 12, 18, and 24 months). The final patient completed their 24-month assessment visit in September 2019, at which time data analysis and manuscript preparation began.
Data Collection and Sources
Patients completed the screening, baseline, and all follow-up assessment visits within their primary care clinic with assistance as needed from research study staff embedded in the clinic. The same level of assistance completing assessment visit materials was provided to both ILI and UC patients. All participants were given a $75 stipend electronically loaded onto ClinCards (Greenphire) at the completion of the baseline visit and each follow-up visit as compensation for their time and travel. Patient follow-up data were collected, regardless of whether or not patients attended intervention sessions. We defined the ideal follow-up visit window as ±2 weeks from the exact projected follow-up visit date (ie, exactly 6 months after the baseline visit date ±2 weeks); however, patients could be seen up to 3 months past the exact projected follow-up visit date (ie, exactly 9 months after the baseline visit date for a 6-month visit).
Rigorous methods were used to maximize patient retention at each assessment visit. Retention procedures focused on increasing the number of contacts between study staff and patients and tailoring strategies to each individual, as these approaches are commonly used in studies with high retention rates.54 Briefly, the next follow-up visit was scheduled at the end of the current visit, and patients were given an appointment reminder card listing the visit's date and time. Patients were contacted approximately 1 month before their follow-up visit to confirm or change the appointment. If the confirmation attempt was unsuccessful, staff contacted patients multiple times using various provided contact methods (eg, phone calls, emails, text messages) beginning with a patient's preferred method(s) and at different times of day/days of the week, depending on the patient's schedule. A similar process was used to schedule follow-up visits with patients who had not yet scheduled their next visit. Patients were reminded of their appointment approximately 1 to 4 days before their visit. Immediately upon a patient not arriving for their scheduled follow-up visit, staff contacted them multiple times using the process previously described. For patients who were difficult to reach or for whom we had outdated contact information, several strategies were used: (1) clinic personnel were asked for updated contact information or assistance locating the patient; (2) the patient's alternate contact was used; and (3) a series of letters from the study's coordinating center were mailed to the patient's current home address.
To further increase contact, patients were mailed birthday and holiday cards each year as well as personalized “thinking of you” cards if the patient reported difficult life circumstances. The previously mentioned newsletters also served to maintain contact and aid in the retention of patients in the UC group, who interacted less with study staff than did those in the ILI group.20 Finally, annual training sessions stressed the importance of retention, and staff members shared ways in which they were able to retain and reengage patients who were not regularly attending visits.20
Patients who withdrew from the study were asked to provide the reason they did so, and common reasons included no longer being interested, time commitment, dissatisfied with group assignment, moved/relocated, change in health status, pregnancy, and personal issues. Additionally, several patients did not provide a reason for their wish to withdraw. Clinic personnel assisted with ascertaining reasons for patients who became lost to follow-up.
Analytical and Statistical Approaches
Primary Aim
To test the effectiveness of a pragmatic, high-intensity lifestyle–based obesity treatment program (ILI) delivered within primary care clinics.
The primary aim consisted of 2 hypotheses that we analyzed using the same approach. The first hypothesis was that the ILI group would have greater percentage reductions in body weight (primary outcome) than would the UC group. The second hypothesis was that the ILI group would have significant improvements in the secondary outcomes (QOL, functional capacity, satisfaction with medical care, and obesity comorbidities) compared with the UC group. To address the primary aim for hypothesis 1, we analyzed weight loss as the mean percentage of baseline weight change at months 6, 12, 18, and 24 in the context of linear mixed-effects multilevel models, which included random cluster (clinic) effects.
In addition to study group (ILI or UC), clinic, assessment time point, and a study group-by-time interaction term, the primary analytic model included patient-level (age, sex, race) variables as explanatory covariates. Clinic-level (clinic size, % African American, % Medicaid/Medicare) variables were explored as potential covariates but were not included in the main model, as they were not statistically significant. Preliminary analytical findings were used to choose the most plausible and efficient repeated-measures covariance structure (eg, compound symmetric, autoregressive, unstructured), at which point the unstructured covariance structure was selected. Although substantial effort was employed to minimize missing data, it was inevitable that some missing data occurred (additional details below). An intention-to-treat analysis that included all randomized patients, regardless of the number of assessments obtained, was conducted. The primary analysis used the mixed-effects model mentioned above and employed restricted maximum likelihood using all available data.
To address the primary aim's second hypothesis, the same analytic methodology was applied to each outcome variable (ie, changes in body weight in kilograms, waist circumference, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, blood glucose, IWQOL total and scale scores, PROMIS-29 scale scores, and patient satisfaction with PCP). Estimates are presented as adjusted least-squares mean changes in each outcome variable within the intervention groups and the difference in adjusted least-squares means between the UC and ILI groups, with estimate precision represented by the 95% CI.
In addition to the main model to address the primary aim, we conducted several sensitivity analyses to examine (1) clinic-level covariates (clinic size, % African American, % enrolled in Medicaid), (2) unadjusted models and models adjusted for patient- and clinic-level covariates, and (3) the effect of combining 2 UC clinics with low enrollment with a UC clinic in close geographic proximity. The sensitivity analyses were conducted using the same analytic strategy used for all primary aim analyses.
Additional Analyses to Test the Secondary Aims
Secondary aim 1
Evaluate relationships between adherence to intervention components (physical activity, diet, sessions, etc) and corresponding changes in body weight and secondary outcomes.
The first secondary aim was accomplished by conducting correlation analyses of changes in body weight percentage and patient-reported outcomes with the percentage of intervention material received, changes in daily minutes of physical activity, and changes in fat intake from baseline to 24 months. The results are presented as correlation coefficients and their associated P values (see Table 9). Additionally, changes in daily minutes of physical activity and percentage dietary fat intake at 6, 12, and 24 months were analyzed in the context of linear mixed-effects (fixed and random) multilevel models using the same analytic approach described for the primary aim. The results are presented as adjusted least-squares mean changes in physical activity and fat intake within the intervention groups and the difference in adjusted least-square means between the UC and ILI groups with estimate precision represented by the 95% CI (see Appendix A, Supplementary Table S5). Subanalyses in different adherent groups, such as among patients who received at least 80% of intervention materials, were also conducted to inform this secondary aim (see Appendix A, Supplementary Table S6). The effect of the number of intervention session materials received on percentage weight loss at 24 months was assessed by analyzing subgroups of ILI participants who received <80% of materials and ≥80% of intervention materials. This analysis was conducted using linear mixed-effects multilevel models using a similar analytic approach as the primary aim; however, these analyses were conducted within the ILI group only. The results are shown as 24-month least-squares mean changes in percentage body weight, body weight in kilograms, and waist circumference between ILI participants who received <80% of materials and ≥80% of intervention materials and the associated 95% CI. Finally, categorical weight loss was analyzed by comparing the percentage of patients achieving ≥5% and ≥10% weight loss at each assessment time using generalized linear mixed models. The results are shown graphically along with the 95% CI (see Figure 4).
Table 9
Relationships Between Intervention Components (Columns) and Corresponding Changes in Body Weight and Secondary Outcomes From Baseline to 24 Months (Rows) Among Patients in the ILI Group.
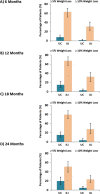
Figure 4
Percentage of Patients Achieving ≥5% and ≥10% Weight Loss From Baseline at 6 Months (A), 12 Months (B), 18 Months (C), and 24 Months (D)a.
Secondary aim 2
Examine the effects of the intervention on system-level practices and patient satisfaction with care.
The second secondary aim was accomplished using a similar strategy as the primary aim, with patient satisfaction with care as the outcome variable of interest in linear mixed-effects multilevel models. The results are presented as the adjusted least-squares mean changes in patient satisfaction with their PCP at 24 months within the UC and ILI groups, as well as adjusted least-squares mean differences between the UC and ILI groups in patient satisfaction with their PCP (see Table 10). Estimate precision is represented by the 95% CI. Due to low survey completion rates among PCPs (see the Results section for details), analyses of system-level practices (ie, PCP weight management practices) were not conducted.
Table 10
Change in Patient Satisfaction With Their PCP From Baseline to 24 Months, Shown as Mean (95% CI).
Secondary aim 3
Test the HTE across clinics and across subgroups of patients (men vs women, White vs African American, older vs younger adults).
Analyses of HTE were an important aspect of this study, as the identification of systematic differences in response to the intervention is important for broad dissemination of our weight loss model and for further tailoring the intervention to specific subgroups. We conducted 3 confirmatory HTE analyses within 3 subgroups: (1) African American and other races; (2) men and women; and (3) younger (21-42 years), middle-aged (43-56 years), and older (57-74 years) adults. Age categories were determined by separating the sample into 3 equal groups based on age. The intervention effect on weight loss in each of the subgroups was investigated using a subgroup variable in linear mixed-effects multilevel models analogous to the primary aim analysis with a focus on intervention-by-subgroup interactions. Changes in percentage weight loss at 6, 12, 18, and 24 months within each subgroup are presented graphically as adjusted least-squares means (see Figure 5). HTE results are also shown as the differences in adjusted least-squares means between the UC and ILI groups for 24-month changes in percentage weight loss, body weight expressed in kilograms, and waist circumference within each subgroup, along with the 95% CI.
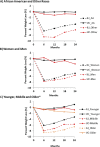
Figure 5
Mean Percentage Change in Weight From Baseline a in African American and Other Races (A), Women and Men (B), and Younger, Middle, and Older Adults (C).
Adverse events
Chi-square tests conducted overall and at each time point were used to compare SAEs between groups. All tests were 2 sided, and P < .05 was used as the cutoff for statistical significance for the primary outcome. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc).
Handling of missing data
Missing data were expected due to dropouts and missed visits. Weight loss was a subject-level variable, and missing assessments would influence tests relative to treatment-by-time interactions. The analyses described above employed restricted maximum likelihood using all data on each participant (ie, data were not deleted in a case-wise manner if they were missing an observation). The model assumes that missing values are missing at random, and all parameters presented in the tables and figures are model-based estimates. To assess the robustness of the results and how sensitive they were to how missing data were handled, we analyzed weight loss using (1) all patients irrespective of missing data to perform analysis of variance (ANOVA) using mixed-effects multilevel models with repeated measures; (2) only patients who completed all assessment visits and had no missing data, to perform ANOVA employing mixed-effects multilevel models with repeated measures; (3) all patients, irrespective of missing data, to conduct analyses using mixed-effects multilevel time trend models with random coefficients to capture individual time trends (does not require complete data across time); and (4) multiple imputation. The results did not appreciably differ according to how the data were analyzed under these 4 different scenarios; thus, the final analyses included all patients irrespective of missing data.
Changes to the Original Study Protocol
Several changes to the original study protocol were made during the study period due to various challenges. All changes were reviewed and approved by Pennington Biomedical's IRB and by PCORI, with formal contract modifications executed as necessary.
Changes to Inclusion/Exclusion Criteria
In December 2016 (8 months into recruitment), the following changes were made to the inclusion criteria:
- The BMI upper range for the trial was increased from 45.0 to 50.0.
- The “recent weight loss” criterion was changed from “within last year” to “within the last 6 months.”
- The blood pressure exclusion criterion of stage 2 or 3 hypertension (systolic ≥160 mm Hg or diastolic ≥100 mm Hg) was removed.
These changes were made because we were excluding many potential patients, which impeded the study's recruitment and made it difficult for PCPs referring patients to the trial. Additionally, patients who were being excluded based on the previous criteria could potentially benefit from the intervention, given that the low-income patient population did not have access to other weight loss options, such as bariatric surgery or pharmacological interventions. These changes removed a significant barrier to recruitment and allowed more patients to participate in the study.
Before recruitment, we also clarified in the protocol that patients who were unable to correctly read 1 or both of the REALM health literacy assessment's practice words (flu and pill) would be deemed ineligible for PROPEL due to an inability to provide written informed consent and the intervention's reliance on written materials. Six patients (3 ILI, 3 UC) were unable to correctly read the REALM's practice words (flu and pill) and were excluded from participation.
Change in Sample Size
In April 2017 (1 year into recruitment), the study's target sample size was reduced from 1080 to 800 patients to meet recruitment targets and reduce the burden on study staff and the patient population. Initially, the trial was powered at 90% power for the subgroup analyses (weight loss by age, sex, and race). We recalculated the sample size estimates based on a traditional level of 80% power for the subgroup analyses, which ensured at least 97% power for the primary analysis if we enrolled 768 patients. This change was approved by the data and safety monitoring board (DSMB) and by PCORI before we made the change in the protocol.
Changes to Assessment Procedures
Before the start of recruitment, several changes were made to the assessment procedures, and these changes are reflected in the revised protocol. Several of these changes were informed by a pilot study conducted during the PROPEL study's development to determine patient acceptability of tablet technology and questionnaires. The pilot study involved 30 patients and was conducted in a primary care clinic that served a population similar to that in the main study. The patients in the pilot study completed several questionnaires on a tablet computer with assistance from study staff as needed.
Based on the feedback obtained in the pilot study, we omitted the Subjective Numeracy Questionnaire, which assessed the ability to perform various mathematical tasks and preference for the use of numeric vs prose information. The questionnaire was removed following our pilot study in which patients responded negatively to the survey. The PROPEL pilot also determined that direct data entry using tablet computers was not suitable for the patient population; thus, this method of data collection was removed from the original protocol. The physical activity assessment tool was changed from the Modifiable Activity Questionnaire to the IPAQ-SF, as investigators determined that this survey was better suited to the anticipated study population.
Logistical Changes
Logistical challenges required changing the data measurement and collection schedule. The original protocol specified that assessment scales and stadiometers would be calibrated monthly by study staff, but the calibration frequency was changed to occur at periodic site monitoring visits. Additionally, remote intervention session monitoring for quality assurance was added in lieu of strictly in-person monitoring. Originally, we planned to collect a finger-stick blood sample for cardiovascular disease risk factor measurement at baseline and all follow-up visits, but due to cost challenges, we limited the finger stick to baseline and the 12- and 24- month visits. These time points were chosen so that the measurements would be evenly spaced in time across the 24-month trial. Several questionnaires were administered at the screening visit rather than the baseline visit to reduce the patient's burden at baseline, and the Food Security Scale Questionnaire was removed from the 6-month assessment to reduce the patient's burden at that visit. The waist circumference measurement directions were changed from taking the measurement with the patient's arm resting at their sides to their arms slightly forward at a 45° angle. This change allowed assessment technicians to more easily read the measurement. The timing of blood pressure measurements was altered slightly, from the patient sitting for 5 to 10 minutes before the first reading to sitting for at least 5 minutes. Originally, patients in the UC group were presented with information on various topics of interest through a series of social meetings, but attendance at these meetings was poor. Therefore, the social meetings were stopped in favor of informational newsletters delivered to UC patients by email or postal mail.
Patient Protection
Several changes were made to the original protocol to ensure proper patient protection against risks. With approval from PROPEL's DSMB, several categories of AEs viewed as expected and unrelated to the study were excluded from AE reporting (unless they met the definition of an SAE), including constipation, joint/muscle pain, and fall-related injuries (other than those related to a secondary condition such as vertigo). Further, a protocol section was added specific to the handling of patients who became pregnant or developed a serious condition during the course of the trial. Finally, blood pressure and blood glucose alert and emergency values were changed in accordance with recognized standards (which changed midway through the trial), and instructions were added.
Focus Group Methods
Study Overview
Before completing the study protocol or intervention curriculum for the analytical aims of the PROPEL study, we conducted a series of focus groups to inform development of the intervention and patient-reported outcomes. Detailed focus group methods and results were previously published by our group, and the information in this section is derived from the publication.21
Participants
Patients as well as physicians or nurse practitioners (here referred to as health care providers) aged 20 years or older, willing to provide written informed consent, and able to speak and understand English were eligible to participate in 1 of 5 structured focus groups held across Louisiana. We solicited participants with an interest in discussing weight loss and weight management strategies. Individuals with a cognitive impairment that would interfere with participation in a group discussion were not eligible.
Convenience sampling was employed, and participants were recruited by word of mouth or from flyers posted within primary care clinics. Written informed consent was obtained before the start of each focus group discussion. Each participant received a $50 gift card. The focus group study protocol, procedures, and consent form were reviewed and approved by the Pennington Biomedical Research Center IRB.
Design and Procedures
The nominal group technique (NGT), a qualitative method of data collection, was used within the focus groups to engage patients and health care providers to obtain their perspective on potential obesity treatment strategies.55-60
The multistep NGT design is useful for systematically stimulating meaningful statements among participants by gathering equally weighted responses to a specific question that tends to offer a valid representation of group views.61-64 The NGT method eliminates the need for audio recording and transcription because verbatim responses are written on a flipchart. Before conducting NGT sessions, the investigative team articulated the specific question and pilot-tested it with individuals desiring to lose weight to ensure that it would capture the responses intended.
Primary care patients residing in 4 communities (Franklin, Bossier City, New Orleans, and Shreveport, LA) and health care providers in Baton Rouge, Louisiana, participated in 1 of 5 NGT focus groups. All sessions were conducted during July 2015. Each group consisted of 6 to 8 participants and included both men and women. Each group session lasted approximately 90 minutes.
Demographic information was collected using survey instruments that included age, race/ethnicity, sex, education, employment, annual household income, and marital and health status. In addition, height and weight were self-reported, and health care providers selected the number of years in their primary occupation as a health care provider.
Preliminary probing questions included, “How do you define overweight?” “What are the main reasons that you would want to lose weight?” “What are the best ways to lose weight?” “What are the major things stopping people from losing weight in your community?” and, “If researchers were successful in designing a weight management program, what would that look like?” The facilitator, accompanied by a cofacilitator, then posed the main question to patients: “What has your doctor or other health care professional done to try to help you lose weight?” Similarly, the health care providers were asked, “What are the main things that you have done to help patients lose weight?”
Participants worked independently to write down as many responses to the question as possible in short phrases that represented their individual views. Participants were then asked to share their answers while the cofacilitator wrote each response verbatim on a flipchart without discussion. Each recorded response was then discussed for the sole purpose of clarification and not for evaluation or debate as to its relative importance. Finally, during the voting phase, participants privately selected what they considered to be the top 3 responses likely to have the greatest impact on losing weight and the top 3 responses that would be the easiest to implement for weight management. A ranking of 3 was given to the most impactful/easiest to implement, and a ranking of 1 was given to the least impactful/most challenging. The facilitator recorded the votes on the flipchart and tallied the votes for each response. The primary results were the top 3 strategies identified in each group; the secondary results were all other ideas. Through an iterative process, the facilitators categorized responses into common themes until consensus was obtained.
Results
Focus Group Results
A series of 5 focus groups (4 for patients, 1 for health care providers) was held to inform the intervention's development; the results were previously published.21 Selected demographic characteristics of the 34 focus group participants (28 patients, 6 health care providers) are shown in Appendix A, Supplementary Table S3. The average BMI was 46.5 for patients and 26.9 for health care providers.21 Eighty-three percent of the health care providers were physicians, and 67% had been in their profession more than 10 years.21
When patients were asked how they defined overweight, they responded that it meant having low self-esteem, and the main reasons patients said they want to lose weight are because they are concerned about their health, have family history issues, and want to have enough energy to keep up with their grandchildren. Patients stated that the best ways to lose weight are controlling portions, planning meals, keeping healthy choices available, keeping a food and exercise diary, and joining a social support group. In addition, patients perceived that a lifestyle change is needed to lose weight, because many had taken diet pills and know they work only temporarily. The most common weight loss barrier cited was the cost of healthy foods, especially for patients with limited incomes. Patients reported that effective weight loss programs should be structured and based on individual needs, because one size does not fit all.
The most consistent things health care providers reported doing to help patients lose weight were counseling them on appropriate portion size, advising them to consume fewer calorie-dense foods, encouraging them to read labels, and discussing comorbidities of excess weight. Health care providers perceived the major barriers to losing weight to be the influence of family members, seeing discouraging results, lack of access to healthy foods, staying at different homes, and consuming fast food. Health care providers perceived successful weight management programs to be multidisciplinary, structured, and easily adaptable, as one size does not fit all. They reported not having enough time to spend with patients; 15-minute visits are not conducive to combating the obesity problem.
Four NGT focus group sessions were conducted with patients at 4 clinical sites. Patients generated responses to the question, “What has your doctor or other health care professional done to try to help you lose weight?” Four themes were identified in response to this question. Patients reported that their doctors referred them to exercise more often, referred them to a nutritionist, referred them to take medication, and provided advice only. Table 3 lists the themes, with patient responses from each clinical site given under each theme.
Table 3
Patient Responses: What Has Your Doctor or Other Health Care Professional Done to Try to Help You Lose Weight?
Referred to nutritionist, referred to medication, and referred to exercise were chosen as the ideas most likely to have the greatest impact on losing weight. As the easiest method to implement, patients chose support group, medication referrals, and nutritionist referrals in Bossier City, Franklin, Shreveport, and New Orleans, respectively.
In the fifth NGT focus group session, health care providers generated responses to the question, “What are the main things that you have done to help patients lose weight?” Four themes were identified: referred to nutritionist, referred to metabolic medications, referred to exercise, and referred to weight loss programs. In Table 4, the themes are listed in bold, with provider responses listed under each theme.
Table 4
Health Care Provider (n = 6) Responses: What Are the Main Things That You Have Done to Help Patients Lose Weight?
The top 3 themes that health care providers identified as having the greatest impact on patients losing weight were (1) referred to nutritionist (chosen as easiest to implement), (2) referred to metabolic medications, and (3) referred to exercise.21
Intervention Study Results
Recruitment and Retention
Detailed information on recruitment20 and retention22 was described previously. The flow of participants from initial screening to enrollment (at baseline) is shown in Figure 3.20 A total of 1958 patients were screened for initial eligibility at the 18 randomized clinics. Of those patients, 362 declined participation, 669 did not meet the inclusion/exclusion criteria, and 124 were not enrolled, as their screening visit had not been completed at the time recruitment ended. Further, 102 patients were deemed ineligible and not allocated to a study group due to not having a PCP at a participating clinic. The final sample size was 803 (452 ILI, 351 UC). The final number of patients enrolled per clinic ranged from 2 to 89, with a median of 40.5 patients per clinic.22

Figure 3
Patient Flow Through the PROPEL Trial.
All clinics completed participation between April 2016 and September 2019.22 Eighty-three percent of the sample (80.1% of ILI, 87.7% of UC) had body weight data available at the 24-month visit (Figure 3). Thirty-six patients (n = 19 in ILI, n = 17 in UC) became pregnant, had weight loss surgery, developed a major medical condition, or died during the trial, and their data were censored at a prior visit. The exclusion of these patients resulted in 87.4% (83.6% of ILI, 92.2% of UC) of the sample having weight measured at 24 months.22
Descriptive Characteristics
Table 5 provides the descriptive characteristics of the sample at baseline. Approximately 67% of the sample was African American, and 41.2% reported an annual household income below $20 000.22 The mean age of the sample was 49.4 years, with a range of 21 to 74 years. The mean BMI was 37.2.20 A total of 30.8% of the sample had low literacy, and 30.8% had food insecurity.22 The 2 cluster randomized groups were well balanced with respect to baseline characteristics; however, there was a greater proportion of African American patients and women in the ILI group, and a greater proportion of patients with diabetes in the UC group (Table 5).20
Table 5
Clinic and Participant Characteristics at Baseline.
Primary Aim
To develop and test the effectiveness of a 24-month, patient-centered, pragmatic, and scalable obesity treatment program delivered within primary care in an underserved population.
Hypothesis 1
Relative to patients who receive UC, patients who receive the ILI will have greater percentage reductions in body weight.
Percentage weight loss at 24 months was significantly greater in the ILI group (−4.99% [95% CI, −6.02% to −3.96%]) than in the UC group (−0.48% [95% CI, −1.57% to 0.61%]), with a mean difference of −4.51 percentage points (95% CI, −5.93 to −3.10) between the groups (P < .0001).22 The greatest weight loss occurred at 6 months; however, clinically significant weight loss was maintained at 24 months (Table 6). The ILI group regained an average of 30% of their initial 6-month adjusted weight loss (2.38 of 7.81 kg) by month 24, and approximately 51% of ILI participants maintained at least 5% weight loss at 24 months. Similar results were obtained for changes in absolute weight and waist circumference. Age, sex, and race were included as covariates in all models. Clinic-level covariates (clinic size, % African American, % enrolled in Medicaid) were not significant covariates and were not included in the final models. Appendix A, Supplementary Table S4 provides the results from unadjusted models and models adjusted for patient- and clinic-level covariates. The results from all models were similar.22 The intraclass correlation coefficient (ICC) was calculated as . For percentage weight loss at 24 months, the calculated ICC was 0.14.
Table 6
Changes From Baseline in Weight Loss Variables Over 2 Years, Shown as Mean (95% CI).
Categorical weight loss results at 6, 12, 18, and 24 months are presented in Figure 4.22 At 24 months, 19.62% (95% CI, 12.92%-28.66%) and 4.74% (95% CI, 2.61%-8.45%) of the UC group experienced ≥5% and ≥10% weight loss, respectively, while 50.70% (95% CI, 39.79%-61.55%) and 23.25% (95% CI, 16.52%-31.67%) of the ILI group experienced ≥5% and ≥10% weight loss, respectively.22
Hypothesis 2
Relative to patients in UC, patients in the ILI group will have significant improvements in HRQOL, functional capacity, satisfaction with medical care, and improved obesity comorbidities.
Table 7 describes changes in patient-reported outcomes (QOL and satisfaction with medical care) over 2 years.22 The ILI group showed significant improvements across all 5 weight-related QOL scales and the total score measured by the IWQOL at 6, 12, and 24 months. Changes in the total weight-related QOL score and 4 of the 5 scales (physical function, self-esteem, sexual life, and work) were significantly greater in the ILI group than in the UC group at 6, 12, and 24 months (mean differences P < .05). The mean difference in total weight-related QOL between the 2 groups was 7.53 (95% CI, 5.18-9.88) at 6 months, 7.59 (95% CI, 5.15-10.03) at 12 months, and 6.66 (95% CI, 4.10-9.21) at 24 months, all in favor of the ILI group.
Table 7
Changes From Baseline in Patient-Reported Outcomes Over 2 Years, Shown as Mean (95% CI).
Of the 8 generic HRQOL scales measured by the PROMIS instrument, fatigue, sleep, and social functioning significantly improved in the ILI group at 24 months. There was no change in any of the subscales in the UC group at 24 months. The mean difference between the UC and ILI groups was statistically significant in favor of the ILI for 6 generic QOL scales (Anxiety, Fatigue, Pain Interference, Physical Functioning, Sleep Disturbance, Social Functioning) at 6 months, 3 scales (Pain Interference, Physical Functioning, Social Functioning) at 12 months, and 2 scales (Fatigue, Social Functioning) at 24 months. No significant differences between the 2 groups were identified for the pain intensity or depression scales at any time point. There was no significant difference in patient satisfaction with their PCP between or within the 2 groups at 24 months.
Table 8 describes changes in cardiometabolic risk factors over 2 years.22 In the ILI group, fasting glucose levels decreased significantly from baseline to 12 months but did not change significantly from baseline to 24 months, leading to a mean difference of −7.14 mg/dL (95% CI, −12.12 to −2.16 mg/dL) at 12 months and −0.91 mg/dL (95% CI, −6.28 to 4.45 mg/dL) at 24 months in favor of the ILI group. HDL cholesterol levels increased significantly in the ILI group by 4.54 mg/dL (95% CI, 3.21-5.87 mg/dL) at 12 months and 4.16 mg/dL (95% CI, 2.78-5.54 mg/dL) at 24 months; they did not change in the UC group at 12 or 24 months. The mean difference in HDL cholesterol between the 2 groups was 4.04 mg/dL (95% CI, 2.41-5.68 mg/dL) at 12 months and 4.60 mg/dL (95% CI, 2.88-6.32 mg/dL) at 24 months, both in favor of the ILI group. There were no significant differences in systolic or diastolic blood pressure, LDL cholesterol, or triglycerides between the 2 groups at any time point.
Table 8
Changes From Baseline in Cardiometabolic Disease Risk Factors Over 2 Years, Shown as Mean (95% CI).
Secondary Aims
Secondary aim 1
Evaluate relationships between adherence to intervention components (physical activity, diet, session attendance) and corresponding changes in body weight and secondary outcomes.
Correlations between adherence to intervention components and changes in outcomes at 24 months are presented in Table 9. Overall, weak correlations were identified between weight change at 24 months and intervention components (24-month changes in daily physical activity, change in percentage fat intake, and units of session material received). Change in total daily minutes of physical activity was significantly negatively correlated with percentage weight change, such that increases in physical activity were associated with greater weight loss. Also, the ILI group significantly increased their total daily physical activity at 6 and 12 months, although participants engaged in significantly more activity than did those in the UC group only at 6 months (19.64 minutes/day; 95% CI, 5.76-33.51 minutes/day; Appendix A, Supplementary Table S5). Change in percentage energy from fat was significantly positively correlated with percentage weight change and change in waist circumference and glucose levels, and it was negatively correlated with select metrics of weight-related QOL. Further, the ILI group significantly decreased their percentage energy from fat compared with the UC group at all time points (24 month difference: −2.09%; 95% CI, −3.31% to −0.87%; Appendix A, Supplementary Table S5). The percentage weight change in ILI participants who received ≥80% of the intervention session materials was −7.07% (95% CI, −8.58% to −5.56%) at 24 months, compared with −1.93% (95% CI, −3.81% to −0.06%) among those who received <80% of the sessions (Appendix A, Supplementary Table S6).
Additionally, a greater number of units of intervention session material received was associated with lower waist circumference and higher HDL cholesterol at 24 months. The relationships between the number of intervention session materials received and generic QOL metrics were mixed, while significant positive correlations were found for the overall and subscales of weight-related QOL. Health coaches also tracked their intervention-related contacts with ILI participants outside the 43 sessions included in the intervention curriculum (Appendix A, Supplementary Table S7). Outside the scheduled sessions, coaches had nearly 8000 additional contacts with their patients during the trial. Additional contacts occurred via a variety of communication methods, with text messages being the most used (n = 6852).
Secondary aim 2
Examine the effects of the intervention on system-level practices and patient satisfaction with care.
Patient satisfaction with their PCP did not significantly change within the UC or ILI groups at 24 months relative to baseline (Table 10). Furthermore, there was no significant difference in patient satisfaction with care between the UC and ILI groups at 24 months. In total, 79 PCPs across the 18 clinics enrolled at least 1 patient in the PROPEL trial. Low PCP completion rates of 51.9% (n = 41) and 22.8% (n = 18) were observed for surveys administered at the start and end of the trial, respectively. These modest completion rates precluded multilevel modeling of intervention effects on system-level practice.
Secondary aim 3
Test the HTE across clinics and across subgroups (age, sex, race) of patients.
The results of the 3 planned HTE subgroup analyses are presented in Figure 5 and in Appendix A. The percentage weight loss in the ILI group tended to be lower in African American participants than in those in the “other” race category, and the difference between the ILI and UC groups across all outcomes was at least 1% less at 24 months in African American participants than were outcomes in other races (Appendix A, Supplementary Table S8). Women and men responded similarly to the intervention (Appendix A, Supplementary Table S9); however, differences were observed across age groups. There appeared to be a dose-response association with age, with younger adults experiencing less weight loss in the ILI group than did older adults (Figure 5), although the differences in weight loss between the UC and ILI groups were similar across age groups (Appendix A, Supplementary Table S10).
Two UC clinics failed to enroll more than 4 patients each, and patients at these clinics gained more weight than did those at other UC clinics (Appendix A, Supplementary Figure S1). Therefore, a sensitivity analysis was conducted whereby these patients were combined with those from a UC clinic in close geographic proximity (Appendix A, Supplementary Table S11). The results were similar, with an estimated 24-month weight loss difference of −4.43 (95% CI, −5.86 to −3.00) percentage points between the groups.
Adverse Events
A total of 86 patients had at least 1 SAE during the 24-month intervention (Table 11); 83 patients reported hospitalizations or other life-threatening conditions, and there were 3 deaths. None of the SAEs were related to the intervention. There were no significant differences in SAEs between the groups overall or at any time point.
Table 11
SAEs by Study Group.
Discussion
Context for Study Results
A high-intensity patient-centered lifestyle–based obesity treatment program (ILI) inclusive of 43 sessions and customizable toolbox options delivered by a trained health coach in an underserved primary care population produced clinically significant weight loss over 24 months. A series of focus groups with input from both patients and health care providers contributed to this patient-centered intervention. Patients and health care providers both perceived that the best way to lose weight was to follow a specific plan that included eating healthy foods, exercising, and tracking food consumption. These similarities coincide with previous research in which fruits and vegetables were provided along with a nutrition education component; participants lost an average of 2.0 kg of weight by the end of the study.65
A major hindrance to losing weight perceived by both patients and health care providers was a lack of access to healthy food choices or the associated costs. Despite US supermarkets stocking an average of 28,000 foods, allowing vast food choice,66,67 focus group participants stressed the realities of living on a limited budget with limited access to healthy foods given that fast foods and highly processed foods are less expensive and more readily available than are fresh fruits and vegetables.66 Indeed, approximately 13.5 million people in the United States live in food deserts, which are low-income communities lacking supermarkets or grocery stores that sell affordable, healthy foods.68 In accordance with these findings, the PROPEL ILI group provided meal plans specific to various caloric intake targets and provided food-tracking logs. The intervention was also tailored to be appropriate for both food-secure and food-insecure individuals in various ways. For example, patients were provided handouts depicting low-cost food items appropriate for their meal plan, and health coaches were knowledgeable about budget-friendly food stores in their patients' neighborhoods that stocked these healthy foods.
Patients and health care providers commonly reported that successful weight management programs must be easily adaptable to individual needs because “one size does not fit all.” Weight loss programs often adopt a one-size-fits-all behavioral model that subscribes to the culture of the dominant majority without considering the cultural attitudes and preferences of nonmajority members.69-72 As such, PROPEL implemented a toolbox approach in which the intervention was adapted according to individual patient needs. Toolbox options included keeping a food diary, increasing coach contact, incorporating meal replacements, and wearing a step tracker; these options were decided on via shared decision-making between patients and their health coach.
Finally, patients and health care providers cited referrals to a nutritionist as having the greatest impact on losing weight. Research shows that dietary consultation is one of the most useful services available for weight management; however, health care professionals report their lack of training in nutrition and counseling as a major barrier to offering weight management services to patients with obesity.73-75 In a recent review of strategies to increase obesity management in primary care and overcome such barriers, authors cited the need for health care professions not to “go it alone” but instead to incorporate a multidisciplinary team approach inclusive of nutrition professionals and health coaches.75 The PROPEL trial emphasized this multidisciplinary approach and focus on dietary counseling as an effective weight management strategy, as trained health coaches were embedded within the primary care clinic. Additionally, most PROPEL health coaches had advanced degrees and/or certifications in nutrition, and the intervention relied heavily on nutrition-based information and practices.
Although patients agreed with some of the ideas generated by the health care providers, they indicated that only providing advice does not tangibly assist them in losing weight. Previous research indicated that patients prefer health care providers to give specific recommendations for weight loss, including exercise and dietary instructions.76,77 However, as confirmed by our focus groups, many health care providers feel that they cannot devote adequate clinical time to weight management when faced with acute and chronic demands to manage disease states and illnesses.78,79 Thus, primary care patients may not always feel that their weight concerns are being thoroughly addressed, necessitating a different approach for weight management in primary care.77
Our trial demonstrated the effectiveness of implementing a high-intensity lifestyle–based obesity treatment program that targeted increasing physical activity and reducing dietary intake, consistent with the 2013 AHA/ACC/TOS guidelines, in an underserved primary care population.22 The ILI group experienced an average of 5% weight loss, and approximately 51% of ILI patients maintained at least 5% weight loss at 24 months. Our results are comparable with those from studies conducted in academic health centers, such as the DPP80 and Look AHEAD81 trials, which demonstrated 5.8% and 6.4% weight loss at 24 months, respectively, in their lifestyle intervention groups. Weight loss interventions delivered by PCPs, which have typically been low to moderate intensity, have produced only modest weight loss (1-2 kg).13,82 However, there is mounting evidence that high-intensity lifestyle interventions conducted in primary care can produce greater weight loss (4-7 kg).82 Weight loss percentages in behavioral lifestyle interventions conducted in the POWER trials in primary care clinics in Baltimore, MD; Boston, MA; and Philadelphia, PA, were 5.2%, 1.7%, and 4.7%, respectively, at 24 months.83-85
The PROPEL intervention also led to significant improvements in weight-related QOL in the ILI group compared with UC, reflecting the importance of weight loss to better QOL. There was significant improvement in all subscales and total weight-related QOL at every time point within the ILI group. Additionally, the mean difference between change in scores from the UC and ILI groups was analyzed, and 5 of 6 scales (total weight-related QOL, physical function, self-esteem, sexual life, and work) showed that, relative to baseline, participants in the ILI group had significantly improved QOL compared with those in the UC group at 6, 12, and 24 months. While differences in mean weight loss from baseline between the UC and ILI groups were slightly attenuated at 24 months (−4.5 percentage points) compared with that at 6 months (−6.9 percentage points), little attenuation was observed for weight-related QOL improvements (total score: +7.5 at 6 months vs +6.7 at 24 months), suggesting that QOL changes may be long-lasting. These weight-related QOL changes are clinically meaningful, as represented by the minimal clinically important difference (MCID), which is the smallest difference in a health-related outcome that patients perceive to be beneficial.86 The MCID for the IWQOL-Lite total score was determined to be between 7.7 and 12 in a cohort of patients with severe obesity.86 Our findings for change in total weight-related QOL within the ILI group at all time points, as well as the difference between the ILI and UC groups at all time points except 24 months (difference = 6.6), fall within the aforementioned MCID point estimate range. Similar to our results, participants in the POWER-UP pragmatic weight loss trial reported significant improvements in weight-related QOL at 6 months, which were well maintained through 24 months.89 Thus, lifestyle-based weight loss treatment delivered by a health coach in primary care may yield significant and persistent improvements in weight-related QOL.
Whereas weight-related QOL improved, results were mixed for generic HRQOL. For the PROMIS subscales, the ILI group improved on some scales but experienced no change on others, and improvements were often only at the 6-month time point. None of the generic QOL measures from the PROMIS-29 reached clinical significance at any time point, as defined by the MCIDs reported in samples of patients with chronic pain87 or undergoing spinal surgery.88 MCIDs for the PROMIS-29 QOL instrument have not yet been determined for a sample of patients with obesity in a weight management trial, so the lack of clinical significance for the PROMIS-29 scales should be interpreted with caution, as they were determined for patients with other ailments. However, these findings are consistent with a review of 34 weight loss RCTs that found that generic QOL measures were not consistently improved in weight loss trials, whereas obesity-specific QOL measures were more likely to show improvements within the intervention group.90 Obesity-specific measures, such as the IWQOL, include domains most relevant to people with obesity; thus, they may be more sensitive to change in the context of a weight loss intervention. Our findings agree with this proposition, such that the ILI group had better weight-related QOL than did the UC group at 24 months, while the findings for generic QOL were mixed.
The PROPEL intervention also elicited significant improvements compared with UC in fasting glucose and HDL cholesterol, highlighting the clinical relevance of the 5% weight loss achieved in the ILI group compared with the UC group.22 Specifically, fasting glucose levels decreased in the ILI group at month 12, while fasting glucose did not change significantly in the UC group at either time point, leading to a significant mean difference between the 2 groups at 12 but not 24 months. Further, the ILI group showed beneficial increases in HDL cholesterol compared with the UC group at 12 and 24 months. These changes could be attributed to the increases in physical activity observed in the ILI group. Improvements in HDL cholesterol in the ILI group likely contributed to the observed significant increase in total cholesterol compared with the UC group at 24 months, because LDL cholesterol levels did not change in either group.
Similar to our results, the POWER-UP trial found a significant difference in fasting glucose levels between the enhanced lifestyle intervention group and control group at 12 but not 24 months.83 In contrast to the PROPEL results, POWER-UP found a decrease in fasting glucose levels at both time points in the intervention group, with relatively stable values in the control group, whereas we found the significant difference only at 12 months. The trend of relatively stable glucose values as observed for the ILI group in PROPEL is comparable to that in the DPP, which likewise did not find significant changes from baseline to 24 months in the lifestyle intervention group while showing an increase in the control group; however, the DPP found a significant mean difference in glucose values of approximately 5 mg/dL between the groups at 24 months.91 Look AHEAD found decreases in glucose levels at 12 months in both the lifestyle intervention group and control group, with a significantly greater decrease in the intervention group (approximately −14 mg/dL).92 Further, improvements in HDL cholesterol in the ILI group relative to the UC group in PROPEL were approximately twice as large as the increases in Look AHEAD at 12 and 24 months (approximately +2 mg/dL for both time points);92,93 the DPP and POWER-UP trials did not find a significant difference between the intervention and control group at any time point.83,94 Together, these findings demonstrate the cardiometabolic risk reduction achieved by PROPEL's high-intensity lifestyle intervention (ILI) compared with UC received by patients with obesity.
It is noteworthy that despite the clinically relevant weight loss and accompanying improvements in cardiometabolic parameters in PROPEL, there were no significant improvements in blood pressure at any time point. This differs from Look AHEAD92,93 and DPP,94 which both showed significant reductions in systolic and diastolic blood pressure between the lifestyle intervention group and control group at both 12 and 24 months. Similar to our results, POWER-UP did not find significant changes in blood pressure between the intervention and control groups. The specific reason for this lack of effect in PROPEL is conjecture; however, more than 50% of participants were prescribed antihypertensive medication at baseline and throughout the trial. The concurrent drug treatment along with relatively normal mean blood pressure values at baseline (123/80 mm Hg in the UC group, 123/79 mm Hg in the ILI group), indicating a predominantly well-controlled blood pressure that leaves little room for improvement through the lifestyle intervention, likely masked any blood pressure–lowering effect achieved by the PROPEL intervention.
Subgroup Considerations
We conducted stratified analyses to examine the HTE by race, sex, and age. The results demonstrated that African American participants experienced slightly less weight loss than did participants of other races. These results are consistent with evidence from several multicenter trials,95 such as Look AHEAD, where White participants lost significantly more weight than did African American participants,81 and DPP, where African American women lost less weight than other sex-by-race/ethnicity groups.96 Research has suggested that lower weight loss in African Americans may be mediated by less behavior change and lower engagement with intervention components.97 Given this, and that the psychological predictors of weight loss appear to differ by race,98 behavioral interventions may need to be tailored specifically to African American populations to maximize weight loss. Further, African Americans, especially African American women, experience obesity at a greater rate than do other race/ethnic groups.99 African American cultural norms may influence these obesity rates. It has been documented that African American women tend to be more accepting of larger body sizes.100 Obese African Americans tend to underestimate their actual weight101 and do not believe that health consequences of obesity exist until one is severely obese.102 In addition, African American women report that family members and significant others either do not comment on body weight or do not support weight loss.103 There is also evidence of racial differences in body weight satisfaction/norms; African American women tend to “prefer” higher levels of body weight and have different perceptions of their body shape than do White women.104,105 Additional racial differences exist, with data showing that non-Hispanic White women perceive greater social pressure for weight loss than do African American women.106 These race differences may partially explain the differential response to obesity treatment vis a vis differences in perceived health risks associated with excess body weight and motivations for weight loss. This is an important area for future research.
Although previous studies have demonstrated greater weight loss in men than in women,107 we did not identify any sex differences in our study. Indeed, the percentage weight loss experienced by patients in the ILI group was almost identical in men and women. These results also do not support those from the Look AHEAD study, where men experienced greater weight loss than did women at 2 years.81 The reasons for these disparate results are not clear.
Our results suggest that age may be an important factor in weight loss. Older adults experienced greater weight loss in the intervention group than did younger adults. In the DPP, older age was positively associated with meeting the 7% weight loss goal at the end of the 2-year intervention.108 Similarly, a positive graded association was observed across age groups and weight loss at 2 years in the Look AHEAD lifestyle intervention group.81 In our study, the difference in weight loss between the UC and ILI groups did not differ by age (ie, older adults in the UC also lost more weight than did other age groups); therefore, it is possible that we may have observed some age-related weight loss in the overall cohort of patients.
Generalizability and Implementation of Results
The strengths of this trial warrant discussion.64 A major strength is the diverse sample of clinics and patients from urban and rural regions of Louisiana, which represents a population typically underserved in clinical research. Thus, the results of this trial are broadly generalizable to the large, underserved, low-income population in the United States that faces significant barriers to receiving effective weight loss treatment. Another strength is the incorporation of a health coach into the collaborative care team, which represents a potential model for future weight loss programs in primary care settings. CMS covers intensive behavioral therapy for obesity delivered by a PCP15; however, the sole reliance on PCPs to deliver obesity treatment has limitations. Our results illustrate the effectiveness of a non-PCP (ie, a health coach) delivering obesity treatment based on current guidelines within the primary care setting. Last, our patient-centered approach to all facets of the trial and our detailed retention plan, inclusive of phone calls, text messages, birthday cards, letters, etc, likely contributed to high patient retention rates (83% at 24 months). However, the $75 stipend offered to patients on completion of the assessment visits may have also encouraged high retention, in which case future implementation of a similar program when such stipends are unavailable may lead to lower retention.
Although our program is likely broadly generalizable to primary care patients with obesity, there are potential barriers to future widespread implementation related to patients' lack of financial resources, program engagement, and current health care reimbursement structures. Patients may lack access to healthy, affordable foods, as noted by the high prevalence of food insecurity and low household income among patients in our study. Patients in the PROPEL trial were initially provided healthy food options at no cost, but this may not be an option for future implementation. PROPEL also provided patients with handouts of low-cost healthy food options available in local neighborhood stores along with estimated pricing, a strategy that can be implemented in the future; however, this is a difficult barrier to address fully. Further, underserved populations may have competing demands for their time, including a lack of childcare or transportation and the inability to take time off work to attend their weight management visits within the clinic. PROPEL experienced reasonable intervention engagement, with nearly 70% of ILI patients receiving at least 80% of the study materials; however, the more-engaged patients lost more weight. Therefore, future program success will need to address these barriers in various ways, such as providing transportation to and from the clinic for visits and occasionally providing program coaching sessions by phone or through a virtual platform, according to the patient's needs. Perhaps the largest barrier to widespread implementation is that the PROPEL model relies on a health coach with advanced nutrition knowledge to deliver the program, which is not currently reimbursable by CMS. Out-of-pocket costs are likely to prevent participation by most underserved patient populations. Thus, clinic administrators, PCPs and the collaborative care team, and health care payers all have a role to play in overcoming barriers to the successful implementation of weight management programs in primary care.
Study Limitations
A limitation of the study is that our sample was composed primarily of women (approximately 84%), which limits our ability to examine differences in weight loss across sex-by-race subgroups. However, our study is not unique in this regard, as women have made up an average of 73% of the samples in other lifestyle-based weight loss interventions.22,109 Our study encountered and addressed additional challenges and limitations in implementation that are likely unique to trials conducted in health care settings.
An overarching theme to the challenges incurred relates to the fact that patient care, not research, is the priority within primary care clinics, which necessitates adaptable study practices. Although we did not formally track instances in which the needs of the clinical practice created logistical challenges for the study, we observed this across all 18 clinics. It was essential to the study team, as well as future study teams in health care settings, to minimize the clinic's burden to participate in the research study by recognizing that clinical care is the priority and that study personnel are guests in the clinic. Related to this, we initially experienced slow patient recruitment, which necessitated a decrease in the study's sample size from 1080 to 800 patients. The one-size-fits-all recruitment approach, which relied too heavily on active participation of clinic PCPs and clinical staff, was problematic. Thus, we consulted with clinic personnel, stakeholders, and patient partners to develop clinic-specific recruitment strategies, such as EMR-based approaches and increasing study staff presence and efforts. After we customized the recruitment strategies, PROPEL successfully met the revised recruitment targets, which provided adequate statistical power to detect as little as a 3.5-percentage-point difference between the ILI and UC groups at 24 months.
Finally, low survey completion rates among PCPs (52% at baseline, 23% at 24 months) precluded our intended investigation of practice-level changes; in fact, the 50% baseline survey completion rate reflects the engagement level typically seen for PCPs who have many competing demands for their time.110,111 The low end-of-trial survey response reflects the high PCP turnover observed within the clinics, with the highest turnover observed within FQHCs. Nearly 30% of the PCPs with patients enrolled in PROPEL were no longer employed within the clinic by the end of the trial and were thus lost to our attempts to follow-up with PCPs. The low survey completion rates may also reflect overall low engagement in the trial; however, all PCPs were involved in the recruitment process and provided medical clearance for all enrolled patients. Additionally, we tracked use of the password-protected PROPEL website on which all PCP webinars were posted; only PROPEL PCPs had access. The website indicates moderate engagement, with 140 page views from 40 unique visitors.
Future Research
Our results demonstrate the effectiveness of a high-intensity lifestyle–based obesity treatment program (ILI), consistent with the 2013 AHA/ACC/TOS guidelines, in an underserved primary care population. The ILI group experienced an average of 5% weight loss, and 51% of patients maintained at least 5% weight loss at 24 months. Treatment models based on the AHA/ACC/TOS guidelines that are adapted to real-life settings and that add effective delivery methods for obesity treatment in primary care are needed. The results from the PROPEL trial demonstrate that significant weight loss in primary care is possible by the addition of a health coach to the collaborative care team. These findings support the recommendation for a multidisciplinary approach to weight management in primary care.75
CMS currently reimburses for obesity treatment delivered by a PCP15; however, our results provide support for expanding the eligibility for reimbursement to other members of a collaborative care team who can be trained in obesity treatment approaches such as the one used in this study. Novel reimbursement schemes for obesity treatment are needed to support the implementation of higher-intensity, evidence-based therapies more broadly across health systems. Innovative strategies to implement this approach across different health care settings should be explored using implementation science frameworks.112 Further, given the proliferation of EMRs, electronic scales, and associated technologies in the last 5 years, there is a need to better understand the effectiveness of transitioning intervention delivery to new technologies, such as EMR patient portals, and using telemedicine to deliver virtual sessions.
PROPEL patients may not have accessed the online tools, such as the CTS, as frequently as intended due to various barriers that we did not systematically identify during our trial. Therefore, there is also a need to understand and provide solutions to increase the use of online intervention delivery tools, including patient portals and other telemedicine approaches, among both underserved and older populations. The low survey completion rate among PCPs in our and previous studies elucidates the need for future research to increase PCP engagement in research studies.
Additional barriers to PCP engagement in research and weight management practices should also be explored, including potentially high turnover rates among PCPs in community clinics over time. The webinar series for PCPs in the UC group addressed 1 such barrier, that of biases and stigma associated with obesity. Specifically, the webinar aimed to explore the issue in an effort to improve clinicians' interactions with patients with obesity, which is essential to providing high-quality, equitable health care. Although we did not measure implicit bias in this trial, we recognize it as a major issue in obesity treatment and one that future research should address. Most health care providers do not openly discriminate against their patients with obesity, but their implicit attitudes may guide their behavior and decision-making, which, in turn, may cause their patients to feel disrespect and mistrust.113 These feelings may discourage patients from seeking future care, resulting in poor health outcomes.113 Indeed, a systematic review of 42 articles examining implicit bias in health care professionals revealed that 83% of studies (n = 35) found evidence of implicit biases on the basis of various patient characteristics, including weight.114 All studies that examined correlations found that greater implicit bias among health care professionals was related to lower quality of health care.114 Future trials on weight management in health care settings should use tools, such as the Implicit Associations Test,115 to measure the implicit biases health care professionals may have regarding obesity in an effort to better understand the impact of these biases on health-related outcomes for patients with obesity.
Conclusions
This study addressed the gap between present-day obesity management guidelines and what is currently implemented in primary care.22 Our approach was a treatment model based on the AHA/ACC/TOS guidelines but adapted to real-life settings and coupled with effective delivery methods for obesity treatment in the primary care setting. The results from this pragmatic trial demonstrate that clinically significant weight loss in primary care is possible. We believe that the keystone of this outcome is the addition of a trained health coach to the collaborative care team to administer a high-intensity lifestyle, patient-centered intervention program.
Percentage weight loss at 24 months was significantly greater in the ILI group (−4.99% [95% CI, −6.02% to −3.96%]) than in the UC group (−0.48% [95% CI, −1.57% to 0.61%]), with a mean difference of −4.51% (95% CI, −5.92% to −3.10%) between the groups. Peak weight loss occurred during the first 6 months of the trial; however, clinically significant weight loss was maintained through 24 months. Additionally, we found clinically significant improvements in weight-related QOL in the ILI group compared with the UC group. Although improvements in cardiometabolic risk factors were limited, ILI patients had significantly higher HDL cholesterol than did the UC group at 24 months. Relative to the UC group, the ILI group also had significantly lower blood glucose at 12 months, but this was not sustained at 24 months. In conclusion, in our trial, the implementation of a high-intensity lifestyle–based obesity treatment program consistent with the 2013 AHA/ACC/TOS guidelines produced clinically significant weight loss and improvements in cardiometabolic risk factors and QOL over 24 months in underserved patients in primary care clinics.22
References
- 1.
- Centers for Disease Control and Prevention. BRFSS prevalence & trends data. Updated September 13, 2017. Accessed June 9, 2021. https://www
.cdc.gov/brfss /brfssprevalence/index.html - 2.
- Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35(8):1999-2010. [PubMed: 15272139]
- 3.
- US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-based Report. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2013. Accessed June 9, 2021. http://www
.cdc.gov/uscs - 4.
- Brown JR, O'Connor GT. Coronary heart disease and prevention in the United States. N Engl J Med. 2010;362(23):2150-2153. [PubMed: 20558365]
- 5.
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233-1241. [PubMed: 15769763]
- 6.
- Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195-1200. [PubMed: 11559264]
- 7.
- Sherry B, Blanck HM, Galuska DA, Pan L, Dietz WH, Balluz L. Vital signs: state-specific obesity prevalence among adults—United States, 2009. Morb Mortal Wkly Rep. 2010;59(30):951-955. [PubMed: 20689500]
- 8.
- US Census Bureau. State Population by Characteristics: 2010-2019. 2010 Demographic Profile: Louisiana 2010. Accessed July 19, 2021. https://www
.census.gov /data/tables/time-series /demo/popest/2010s-state-detail .html - 9.
- National Institute for Literacy. The State of Literacy in America: Estimates at the Local, State and National Levels. US Government Printing Office; 1998.
- 10.
- Flegal K, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284-2291. [PubMed: 27272580]
- 11.
- Slack T, Myers CA, Martin CK, Heymsfield SB. The geographic concentration of US adult obesity prevalence and associated social, economic, and environmental factors. Obesity (Silver Spring). 2014;22(3):868-874. [PubMed: 23630100]
- 12.
- US Department of Health and Human Services. Healthy People 2020 Framework: the vision, mission, and goals of Healthy People 2020. 2011. Accessed September 22, 2012. https://www
.healthypeople .gov/sites/default /files/HP2020Framework.pdf - 13.
- Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann NY Acad Sci. 2013;1281:191-206. [PMC free article: PMC3618542] [PubMed: 23323827]
- 14.
- US Preventive Services Task Force. Screening for and management of obesity in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373-378. [PubMed: 22733087]
- 15.
- US Centers for Medicare & Medicaid Services. Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). November 29, 2011. Accessed on February 19, 2018. http://www
.cms.gov/medicare-coverage-database /details/nca-decision-memo .aspx?&NcaName =Intensive %20Behavioral%20Therapy %20for%20Obesity&bc =ACAAAAAAIAAA&NCAId=253& - 16.
- Pagoto SL, Pbert L, Emmons K, Farber D; Society of Behavioral Medicine Public Policy Leadership Group. The Society for Behavioral Medicine position statement on the CMS decision memo on intensive behavioral therapy for obesity. Transl Behav Med. 2012;2(4):381-383. [PMC free article: PMC3717932] [PubMed: 24073141]
- 17.
- Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312(17):1779-1791. [PMC free article: PMC4443898] [PubMed: 25369490]
- 18.
- Jensen MD, Ryan DH. New obesity guidelines: promise and potential. JAMA. 2014;311(1):23-24. [PubMed: 24381963]
- 19.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102-S138. [PMC free article: PMC5819889] [PubMed: 24222017]
- 20.
- Katzmarzyk PT, Martin CK, Newton RL Jr, et al. Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL): rationale, design and baseline characteristics. Contemp Clin Trials. 2018;67:1-10. [PMC free article: PMC5965693] [PubMed: 29408562]
- 21.
- Kennedy BM, Kennedy KB, Sarpong DF, Katzmarzyk PT. Perceptions of obesity treatment options among healthcare providers and low-income primary care patients. Ochsner J. 2016;16(2):158-165. [PMC free article: PMC4896661] [PubMed: 27303227]
- 22.
- Katzmarzyk PT, Martin CK, Newton RL Jr, et al. Weight loss in underserved patients–a cluster randomized trial. N Engl J Med. 2020(383):909-918. [PMC free article: PMC7493523] [PubMed: 32877581]
- 23.
- Federal Office of Rural Health Policy, Health Resources and Services Administration. List of Rural Counties and Designated Eligible Census Tracts in Metropolitan Counties: Updated Census 2010. Updated December 31, 2018. Accessed January 19, 2018. https://www
.hrsa.gov /sites/default/files /ruralhealth/resources /forhpeligibleareas.pdf - 24.
- The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165-2171. [PMC free article: PMC1282458] [PubMed: 12453955]
- 25.
- The Look AHEAD Research Group, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14(5):737-752. [PMC free article: PMC2613279] [PubMed: 16855180]
- 26.
- Rickman AD, Williamson DA, Martin CK, et al. The CALERIE study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32(6):874-881. [PMC free article: PMC3185196] [PubMed: 21767664]
- 27.
- Weiss B, Schwartzberg J, Davis T, Parker R, Sokol P, Williams M. Health Literacy and Patient Safety: Help Patients Understand: Manual for Clinicians. AMA Foundation; 2007. Accessed June 9, 2021. http://www.partnershiphp.org/Providers/HealthServices/Documents/Health%20Education/CandLToolKit/2%20Manual%20for%20Clinicians.pdf
- 28.
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan to Improve Health Literacy. US Department of Health and Human Services; 2010.
- 29.
- Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD Study: factors associated with long-term success. Obesity (Silver Spring). 2011;19:1987-1998. [PMC free article: PMC3183129] [PubMed: 21779086]
- 30.
- Neiberg RH, Wing RR, Bray GA, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD study. Obesity (Silver Spring). 2012;20(10):2048-2056. [PMC free article: PMC3632374] [PubMed: 22327053]
- 31.
- US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans 2010, 7th Edition. US Government Printing Office; 2010.
- 32.
- Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Math Biosci Eng. 2009;6(4):873-887. [PMC free article: PMC2764961] [PubMed: 19835433]
- 33.
- Thomas DM, Das SK, Levine JA, et al. New fat free mass – fat mass model for use in physiological energy balance equations. Nutr Metab. 2010;7:39. doi: 10.1186/1743-7075-7-39 [PMC free article: PMC2879256] [PubMed: 20459692] [CrossRef]
- 34.
- Thomas DM, Martin CK, Heymsfield S, Redman LM, Schoeller DA, Levine JA. A simple model predicting individual weight change in humans. J Biol Dyn. 2011;5(6):579-599. [PMC free article: PMC3975626] [PubMed: 24707319]
- 35.
- Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92(6):1326-1331. [PMC free article: PMC2980958] [PubMed: 20962159]
- 36.
- Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026-1033. [PubMed: 18049342]
- 37.
- Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000. US Department of Agriculture, Food and Nutrition Service; 2000.
- 38.
- Bennett WL, Wang NY, Gudzune KA, et al. Satisfaction with primary care provider involvement is associated with greater weight loss: results from the practice-based POWER trial. Patient Educ Couns. 2015;98(9):1099-1105. [PMC free article: PMC4546866] [PubMed: 26026649]
- 39.
- Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. [PubMed: 12900694]
- 40.
- Fuller NR, Williams K, Shrestha R, et al. Changes in physical activity during a weight loss intervention and follow-up: a randomized controlled trial. Clin Obes. 2014;4:127-135. [PMC free article: PMC4282338] [PubMed: 25826767]
- 41.
- Thompson FE, Midthune D, Subar AF, Kipnis V, Kahle LL, Schatzkin A. Development and evaluation of a short instrument to estimate usual dietary intake of percentage energy from fat. J Am Diet Assoc. 2007;107(5):760-767. [PubMed: 17467371]
- 42.
- Havas S, Heimendinger J, Damron D, et al. 5 A Day for better health--nine community research projects to increase fruit and vegetable consumption. Public Health Rep. 1995;110(1):68-79. [PMC free article: PMC1382077] [PubMed: 7838947]
- 43.
- Thompson FE, Kipnis V, Subar AF, et al. Evaluation of 2 brief instruments and a food-frequency questionnaire to estimate daily number of servings of fruit and vegetables. Am J Clin Nutr. 2000;71(6):1503-1510. [PubMed: 10837291]
- 44.
- Hedrick VE, Savla J, Comber DL, et al. Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): sugar-sweetened beverages and total beverage energy intake. J Acad Nutr Diet. 2012;112(6):840-849. [PMC free article: PMC3379009] [PubMed: 22709811]
- 45.
- Stunkard AJ, Messick S. Eating Inventory Manual. The Psychological Corporation; 1988.
- 46.
- Hargraves JL, Hays RD, Cleary PD. Psychometric properties of the Consumer Assessment of Health Plans Study (CAHPS) 2.0 adult core survey. Health Serv Res. 2003;38(6 Pt 1):1509-1527. [PMC free article: PMC1360961] [PubMed: 14727785]
- 47.
- Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21(6):661-665. [PMC free article: PMC1924639] [PubMed: 16808754]
- 48.
- Befort CA, Van Wormer JJ, DeSouza C, et al. Protocol for the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) trial: comparing three obesity treatment models in rural primary care. Contemp Clin Trials. 2016;47:304-314. [PubMed: 26898748]
- 49.
- Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3-S11. [PMC free article: PMC2829758] [PubMed: 17443116]
- 50.
- Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. [PMC free article: PMC2965562] [PubMed: 20685078]
- 51.
- Kolotkin RL, Crosby RD. Psychometric evaluation of the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11(2):157-171. [PubMed: 12018739]
- 52.
- Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9(2):102-111. [PubMed: 11316344]
- 53.
- Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170(2):146-154. [PubMed: 20101009]
- 54.
- Abshire M, Dinglas VD, Cajita MIA, Eakin MN, Needham DM, Himmelfarb CD. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol. 2017;17(1):30. doi:10.1186/s12874-017-0310-z [PMC free article: PMC5319074] [PubMed: 28219336] [CrossRef]
- 55.
- Pena A, Estrada CA, Soniat D, Taylor B, Burton M. Nominal group technique: a brainstorming tool for identifying areas to improve pain management in hospitalized patients. J Hosp Med. 2012;7(5):416-420. [PubMed: 22190453]
- 56.
- Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents' perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. [PMC free article: PMC2517943] [PubMed: 18612745]
- 57.
- Crenshaw K, Shewchuk RM, Qu H, et al. What should we include in a cultural competence curriculum? An emerging formative evaluation process to foster curriculum development. Acad Med. 2011;86(3):333-341. [PMC free article: PMC3046368] [PubMed: 21248602]
- 58.
- Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health. 1972;62(3):337-342. [PMC free article: PMC1530096] [PubMed: 5011164]
- 59.
- Safford MM, Shewchuk R, Qu H, et al. Reasons for not intensifying medications: Differentiating “clinical inertia” from appropriate care. J Gen Intern Med. 2007;22(12):1648-1655. [PMC free article: PMC2219839] [PubMed: 17957346]
- 60.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537-546. [PMC free article: PMC1496744] [PubMed: 10491242]
- 61.
- Jefferson WK, Zunker C, Feucht JC, et al. Use of the nominal group technique (NGT) to understand the perceptions of the healthiness of foods associated with African Americans. Eval Program Plann. 2010;33(4):343-348. [PubMed: 20047762]
- 62.
- Miller D, Shewchuk R, Elliot TR, Richards S. Nominal group technique: a process for identifying diabetes self-care issues among patients and caregivers. Diabetes Educ. 2000;26(2):305-310, 312, 314. [PubMed: 10865596]
- 63.
- Shewchuk LD, Baracos VE, Field CJ. Dietary L-glutamine does not improve lymphocyte metabolism or function in exercise-trained rats. Med Sci Sports Exerc. 1997;29(4):474-481. [PubMed: 9107629]
- 64.
- Elliott T, Shewchuk R. Problem solving therapy for family caregivers of persons with severe physical disabilities. Behav Res Ther. 2000:309-327.
- 65.
- Kennedy BM, Champagne CM, Ryan DH, et al. The “Rolling Store:” an economical and environmental approach to the prevention of weight gain in African American women. Ethn Dis. 2009;19(1):7-12. [PubMed: 19341156]
- 66.
- Fukuoka Y, Lindgren TG, Bonnet K, Kamitani E. Perception and sense of control over eating behaviors among a diverse sample of adults at risk for type 2 diabetes. Diabetes Educ. 2014;40(3):308-318. [PMC free article: PMC4326591] [PubMed: 24525569]
- 67.
- Food Marketing Institute. Supermarket facts. Accessed July 15, 2021. https://www
.fmi.org/our-research /supermarket-facts - 68.
- Ver Ploeg M, Nulph D, Williams RB. Mapping Food Deserts in the US. Amber Waves; 2011.
- 69.
- Kennedy BM, Ard JD, Harrison L Jr, et al. Cultural characteristics of African Americans: implications for the design of trials that target behavior and health promotion programs. Ethn Dis. 2007;17(3):548-554. [PubMed: 17985512]
- 70.
- Blixen CE, Singh A, Xu M, Thacker H, Mascha E. What women want: understanding obesity and preferences for primary care weight reduction interventions among African-American and Caucasian women. J Natl Med Assoc. 2006;98(7):1160-1170. [PMC free article: PMC2569457] [PubMed: 16895288]
- 71.
- Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK, Kumanyaka SK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53(6 Suppl):1631s-1638s. [PubMed: 2031498]
- 72.
- Kumanyika SK, Morssink C, Agurs T. Models for dietary and weight change in African-American women: identifying cultural components. Ethn Dis. 1992;2(2):166-175. [PubMed: 1467754]
- 73.
- Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians' weight loss counseling in two public hospital primary care clinics. Acad Med. 2004;79(2):156-161. [PubMed: 14744717]
- 74.
- Elson RB, Splett PL, Bostick RM, Aeppli D, Haberman M. Dietitian practices for adult outpatients with hypercholesterolemia referred by physicians. The Minnesota Dietitian Survey. Arch Fam Med. 1994;3(12):1073-1080. [PubMed: 7804492]
- 75.
- Kahan SI. Practical strategies for engaging individuals with obesity in primary care. Mayo Clin Proc. 2018;93(3):351-359. [PubMed: 29502565]
- 76.
- Greiner KA, Born W, Hall S, Hou Q, Kimminau KS, Ahluwalia JS. Discussing weight with obese primary care patients: physician and patient perceptions. J Gen Intern Med. 2008;23(5):581-587. [PMC free article: PMC2324159] [PubMed: 18322760]
- 77.
- Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract. 2001;50(6):513-518. [PubMed: 11401737]
- 78.
- Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38(2):166-171. [PubMed: 8308509]
- 79.
- Jaen CR, McIlvain H, Pol L, Phillips RL Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859-863. [PubMed: 11674888]
- 80.
- Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102-2107. [PMC free article: PMC1762038] [PubMed: 16936160]
- 81.
- Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring). 2011;19(10):1987-1998. [PMC free article: PMC3183129] [PubMed: 21779086]
- 82.
- Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary care interventions for obesity: review of the evidence. Curr Obes Rep. 2019;8(2):128-136. [PMC free article: PMC6520163] [PubMed: 30888632]
- 83.
- Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969-1979. [PMC free article: PMC3282598] [PubMed: 22082239]
- 84.
- Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959-1968. [PMC free article: PMC4074540] [PubMed: 22085317]
- 85.
- Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565-574. [PMC free article: PMC3609656] [PubMed: 22412073]
- 86.
- Warkentin LM, Majumdar SR, Johnson JA, et al. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: two-year prospective cohort study. BMC Med. 2014;12(1):175. doi:10.1186/s12916-014-0175-5 [PMC free article: PMC4212133] [PubMed: 25315502] [CrossRef]
- 87.
- Kroenke K, Yu Z, Wu J, Kean J, Monahan PO. Operating Characteristics of PROMIS four-item depression and anxiety scales in primary care patients with chronic pain. Pain Med. 2014;15(11):1892-1901. [PMC free article: PMC6283354] [PubMed: 25138978]
- 88.
- Purvis TE, Andreou E, Neuman BJ, Riley LH III, Skolasky RL. Concurrent validity and responsiveness of PROMIS health domains among patients presenting for anterior cervical spine surgery. Spine (Phila Pa 1976). 2017;42(23):E1357-E1365. [PubMed: 28742757]
- 89.
- Sarwer DB, Moore RH, Diewald LK, et al. The impact of a primary care-based weight loss intervention on the quality of life. Int J Obes (Lond). 2013;37 Suppl 1(0 1):S25-S30. [PMC free article: PMC3786773] [PubMed: 23921778]
- 90.
- Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58(6):568-578. [PubMed: 15878470]
- 91.
- The DPP Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393-403. [PMC free article: PMC1370926] [PubMed: 11832527]
- 92.
- Look ARG, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374-1383. [PMC free article: PMC2665929] [PubMed: 17363746]
- 93.
- The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. [PMC free article: PMC3791615] [PubMed: 23796131]
- 94.
- Orchard TJ, Temprosa M, Barrett-Connor E, et al. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30(1):46-55. [PMC free article: PMC3524372] [PubMed: 22812594]
- 95.
- Wingo BC, Carson TL, Ard J. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obes Rev. 2014;15 Suppl 4:46-61. [PubMed: 25196406]
- 96.
- West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring). 2008;16(6):1413-1420. [PubMed: 18421273]
- 97.
- Blackman Carr LT, Samuel-Hodge C, Ward DS, Evenson KR, Bangdiwala SI, Tate DF. Racial differences in weight loss mediated by engagement and behavior change. Ethn Dis. 2018;28(1):43-48. [PMC free article: PMC5794447] [PubMed: 29467565]
- 98.
- Jerome GJ, Myers VH, Young DR, et al. Psychosocial predictors of weight loss by race and sex. Clin Obes. 2015;5(6):342-348. [PMC free article: PMC4715521] [PubMed: 26486256]
- 99.
- Ogden CL, Fryar CD, Martin CB, et al. Trends in obesity prevalence by race and Hispanic origin—1999-2000 to 2017-2018. JAMA. 2020;324(12):1208-1210. [PMC free article: PMC7455882] [PubMed: 32857101]
- 100.
- Mama SK, Quill BE, Fernandez-Esquer ME, Reese-Smith JY, Banda JA, Lee RE. Body image and physical activity among Latina and African American women. Ethn Dis. 2011;21(3):281-287. [PubMed: 21942159]
- 101.
- Dorsey RR, Eberhardt MS, Ogden CL. Racial/ethnic differences in weight perception. Obesity (Silver Spring). 2009;17(4):790-795. [PubMed: 19148119]
- 102.
- Baruth M, Sharpe PA, Magwood G, Wilcox S, Schlaff RA. Body size perceptions among overweight and obese African American women. Ethn Dis. 2015;25(4):391-398. [PMC free article: PMC4671444] [PubMed: 26674119]
- 103.
- Baturka N, Hornsby PP, Schorling JB. Clinical implications of body image among rural African-American women. J Gen Intern Med. 2000;15(4):235-241. [PMC free article: PMC1495436] [PubMed: 10759998]
- 104.
- Padgett J, Biro FM. Different shapes in different cultures: body dissatisfaction, overweight, and obesity in African-American and Caucasian females. J Pediatr Adolesc Gynecol. 2003;16(6):349-354. [PubMed: 14642955]
- 105.
- Schuler PB, Vinci D, Isosaari RM, et al. Body-shape perceptions and body mass index of older African American and European American women. J Cross Cult Gerontol. 2008;23(3):255-264. [PubMed: 18379865]
- 106.
- Keith NR, Hemmerlein KA, Clark DO. Weight loss attitudes and social forces in urban poor Black and White women. Am J Health Behav. 2015;39(1):34-42. [PMC free article: PMC4567042] [PubMed: 25290595]
- 107.
- Williams RL, Wood LG, Collins CE, Callister R. Effectiveness of weight loss interventions--is there a difference between men and women: a systematic review. Obes Rev. 2015;16(2):171-186. [PMC free article: PMC4359685] [PubMed: 25494712]
- 108.
- Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426-1434. [PMC free article: PMC2505058] [PubMed: 15483207]
- 109.
- Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring). 2012;20(6):1234-1239. [PubMed: 21633403]
- 110.
- Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15(1):32. doi:10.1186/s12874-015-0016-z [PMC free article: PMC4404667] [PubMed: 25888346] [CrossRef]
- 111.
- Kellerman SE, Herold J. Physician response to surveys: a review of the literature. Am J Prev Med. 2001;20(1):61-67. [PubMed: 11137777]
- 112.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi:10.1186/1748-5908-4-50 [PMC free article: PMC2736161] [PubMed: 19664226] [CrossRef]
- 113.
- Phelan SM, Burgess DJ, Yeazel MW, Hellerstedt WL, Griffin JM, van Ryn M. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obesity Rev. 2015;16(4):319-326. [PMC free article: PMC4381543] [PubMed: 25752756]
- 114.
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi:10.1186/s12910-017-0179-8 [PMC free article: PMC5333436] [PubMed: 28249596] [CrossRef]
- 115.
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74(6):1464-1480. [PubMed: 9654756]
Related Publications
- Kennedy BM, Kennedy KB, Sarpong DF, Katzmarzyk PT. Perceptions of obesity treatment options among healthcare providers and low-income primary care patients. Ochsner J. 2016:16(2):158-165. [PMC free article: PMC4896661] [PubMed: 27303227]
- Katzmarzyk PT, Martin CK, Newton RL, et al. Promoting Successful Weight Loss in Primary Care in Louisiana (PROPEL): rationale, design and baseline characteristics. Contemp Clin Trials. 2018;67:1-10. [PMC free article: PMC5965693] [PubMed: 29408562]
- Katzmarzyk PT, Martin CK, Newton RL, et al. Weight loss in underserved patients: a cluster-randomized trial. N Engl J Med. 2020;383(10):909-918. [PMC free article: PMC7493523] [PubMed: 32877581]
- Myers C, Arnold CL, Davis TC; PROPEL Research Group. Cardiovascular health, adiposity, and food insecurity in an underserved population. Nutrients. 2019;11(6):1376. doi:10.3390/nu11061376 [PMC free article: PMC6628173] [PubMed: 31248113] [CrossRef]
- Denstel KD, Mire EF, Martin CK, et al. Engaging Louisiana primary care clinics in pragmatic research: lessons from the PROPEL trial. J Louisiana Public Health Assoc. 2020;1:55-65.
- Höchsmann C, Dorling JL, Martin CK, et al. Effects of a two-year primary care lifestyle intervention on cardiometabolic risk factors: a cluster-randomized trial. Circulation. 2021;143:1202-1214. [PMC free article: PMC7987882] [PubMed: 33557578]
- Myers CA, Martin CK, Apolzan JW, et al; PROPEL Research Groups. Food insecurity and weight loss in an underserved primary care population: a post-hoc analysis of a cluster-randomized trial. Ann Intern Med. 2021;174(7):1032-1034. doi:10.7326/M20-6326 [PMC free article: PMC8292163] [PubMed: 33683931] [CrossRef]
Acknowledgments
We would like to acknowledge the patients and stakeholders who are members of the PROPEL patient advisory boards, community monitoring board, and the project management committee (Chris Lodge and Ava Zebrick, MSHCM), who significantly impacted the trial's design and conduct. We are indebted to the PROPEL patients, assessment technicians, and health coaches, without whom this study would not have been possible. We gratefully acknowledge the contributions of Willie C. White III, MPH, and the David Raines Community Health Centers; Dr Gary Wiltz and the Teche Action Clinic sites; Michael G. Griffith and Dr Robert Post and Daughters of Charity Services of New Orleans; and the Ochsner Health System and Access Health Louisiana clinic sites. We also thank our DSMB for monitoring patient safety and the overall conduct of the trial: Robert Ross, PhD (chair); John Lefante, PhD; Michael Rolfson, MD; and Chris Lodge. We wish to thank David B. Allison, Andrew W. Brown, Stephanie L. Dickinson, and Lilian Golzarri-Arroyo of the Indiana University School of Public Health-Bloomington for their work reviewing and verifying the statistical methods and results for the primary outcome analyses.
The PROPEL Research Group includes the following:
Pennington Biomedical Research Center: Peter T. Katzmarzyk, PhD (principal investigator); Robert L. Newton Jr, PhD (outcome assessment director); Corby K. Martin, PhD (intervention director); John W. Apolzan, PhD (intervention co-director); William Johnson, PhD (biostatistician); Kara D. Denstel, MPH (project manager); Emily F. Mire, MS (data manager); Robert K. Singletary Jr, MHS; Cheryl Lewis, MPH; Phillip Brantley, PhD; Ronald Horswell, PhD; Betty Kennedy, PhD; and Dachuan Zhang
MAppStats: Stephanie Authement, RD, LDN, MS; Shiquita Brooks, RDN, LDN; Danielle Burrell, MEd, MCHES; Leslie Forest-Everage, MA; Angelle Graham Ullmer, RDN, LDN, MS; Laurie Murphy, RDN, LDN; Cristalyn Reynolds, MA; Kevin Sanders, MS, RDN, LDN; Stephen Bower, MS; Daishaun Gabriel, MHA; Hillary Gahagan, MPH; Tabitha K. Gray, MA; Jill Hancock, MPH; Marsha Herrera; Brittany Molinere; Georgia Morgan, MA; Brittany Neyland; Stephanie Rincones; Deanna Robertson, MA; Ekambi Shelton, MPH; Russell J. Tassin, MS; and, Kaili Williams
Louisiana State University Health Sciences Center at New Orleans: Benjamin F. Springgate, MD
Louisiana State University Health Sciences Center at Shreveport: Terry C. Davis, PhD; and Connie L. Arnold, PhD
Ochsner Health System: Eboni Price-Haywood, MD; Carl J. Lavie, MD; and Jewel Harden-Barrios, MEd
Tulane University Medical School: Vivian A. Fonseca, MD; Tina K. Thethi, MD (medical monitor); and Jonathan Gugel, MD
Xavier University: Kathleen B. Kennedy, PhD; Daniel F. Sarpong, PhD; and Amina D. Massey
The research reported in this article was funded through a PCORI award (OB-1402-10977). The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI or its Board of Governors or Methodology Committee.
Additional support was provided by grant 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health that funds the Louisiana Clinical and Translational Science Center, and by Nutrition Obesity Research Centers grant #P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by National Institute of Diabetes and Digestive and Kidney Diseases.
Our thanks also go to Health and Nutrition Technology (Carmel, CA) for providing us with the HealthOne formula and to Nutrisystem (Fort Washington, PA) for providing us with the meal replacements used in the study. Louisiana State University (LSU), Pennington Biomedical, and Montclair State University have interest in the intellectual property surrounding the weight graph that was used in the intervention, and Corby K. Martin, among others, is an inventor of the technology. Licensing of that technology results in financial benefits to LSU, Pennington Biomedical, Montclair State University, and the inventors.
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Pennington Biomedical. REDCap is a secure web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (OB-1402-10977). Further information available at: https://www.pcori.org/research-results/2014/testing-health-coaching-program-help-patients-obesity-lose-weight-propel-study
Appendices
Appendix A.
Supplementary Tables/Figures (PDF, 415K)
Appendix B.
Intervention Fidelity and Sustainability (PDF, 146K)
Appendix C.
Data Management Plan (PDF, 112K)
Suggested citation:
Katzmarzyk PT, Martin CK, Newton RL Jr, et al. (2021). Testing a Health Coaching Program to Help Patients with Obesity Lose Weight—The PROPEL Study. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/06.2021.OB.140210977
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Testing a Health Coaching Program to Help Patients with Obesity Lose Weight—The ...Testing a Health Coaching Program to Help Patients with Obesity Lose Weight—The PROPEL Study
Your browsing activity is empty.
Activity recording is turned off.
See more...
