NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The 5-alpha reductase inhibitors are a family of agents used to treat benign prostatic hypertrophy. They act by inhibiting the steroid 5-alpha reductase which catalyzes the conversion of testosterone to dihydrotestosterone. Dihydrotestosterone is an important prostatic growth factor and its inhibition causes gradual shrinkage of prostate. Both of the available agents lower serum concentrations of dihydrotestosterone without affecting serum testosterone and usually with minimal antiandrogenic effects.
The 5-alpha reductase inhibitors in clinical use in the United States include two agents of similar chemical structure (azasteroids) and activity: finasteride (Proscar: 1992) and dutasteride (Avodart: 2001). Both agents cause a reduction in the size of the prostate, improve urinary flow and suppress prostate-specific antigen (PSA) levels. Both finasteride and dutasteride are associated with a low rate (<1%) of serum enzyme elevations during chronic therapy, which are usually mild-to-moderate in severity, self-limited in course, and rarely require dose modification or discontinuation. Neither of the 5-alpha reductase inhibitors in current use has been linked to clinically apparent acute liver injury.
The 5-alpha reductase inhibitors used for benign prostatic hypertrophy are discussed individually, with references on their hepatotoxicity given together at the end of this introductory section.
Drug Class: Benign Prostatic Hypertrophy Agents, 5-Alpha Reductase Inhibitors
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dutasteride | 164656-23-9 | C27-H30-F6-N2-O2 |
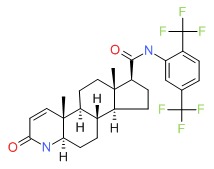 |
| Finasteride | 98319-26-7 | C23-H36-N2-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 09 January 2018
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 555-88.(Expert review of hepatotoxicity published in 1999; the 5-alpha reductase inhibitors are not discussed).
- Chitturi S, Farrell GC. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp 605-20.(Review of hepatotoxicity of sex hormones; the 5-alpha reductase inhibitors are not discussed).
- Snyder PJ. 5-alpha reductase inhibitors. Androgens. In, Brunton LL, Chabner BA, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011. p 1205.(Textbook of pharmacology and therapeutics; 5-alpha reductase inhibitors include finasteride which primarily blocks the type II form of the enzyme and dutasteride which blocks both type I and II forms).
- Finasteride for benign prostatic hypertrophy. Med Lett Drugs Ther 1992; 34: 83-84. [PubMed: 1380630](Brief summary of efficacy and safety of finasteride shortly after its approval in the US; no mention of ALT elevations or hepatotoxicity).
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998; 338: 557-63. [PubMed: 9475762](Controlled trial of 4 year course of finasteride vs placebo in 3040 men with symptomatic benign prostatic hypertrophy; side effects included impotence [8.1% vs 3.7%], breast tenderness and enlargement [0.5% vs 0.1%] and rash [0.5% vs 0.1%]; no mention of hepatotoxicity or ALT elevations).
- Marberger MJ. Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. PROWESS Study Group. Urology 1998; 51: 677-86. [PubMed: 9610579](Controlled trial of finasteride vs placebo in 3270 men treated for 2 years; side effects of finasteride were similar to placebo except for sexually related symptoms, no mention of liver injury or ALT elevations).
- Dutasteride (Avodart) for benign prostatic hyperplasia. Med Lett Drugs Ther 2002; 44: 109-10. [PubMed: 12500154](Brief summary of efficacy and safety of dutasteride shortly after its approval in the United States; no mention of ALT elevations or hepatotoxicity).
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60: 434-41. [PubMed: 12350480](Pooled analysis of 3 trials of dutasteride in 4325 men with symptoms of benign prostatic hypertrophy showing 25% decrease in prostate size; side effects included impotence in ~5%, no mention of hepatotoxicity or ALT elevations).
- Libecco JF, Bergfeld WF. Finasteride in the treatment of alopecia. Expert Opin Pharmacother 2004; 5: 933-40. [PubMed: 15102575](Review of chemistry, pharmacology, metabolism, efficacy for hair growth, and safety of finasteride; no mention of hepatotoxicity or ALT elevations).
- Martínez de Guzmán M, Martínez-Crespo JJ. [Finasteride-induced hepatitis]. Farm Hosp 2006; 30: 385. Spanish. [PubMed: 17298197](Two cases of mild enzyme elevations on long term finasteride therapy for hair growth; 18 and 42 year old men with mild serum ALT elevations [78 and 71 U/L] without symptoms or jaundice, improving on stopping finasteride, one had a positive rechallenge).
- Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5 alpha-reductase inhibitors for the enlarged prostate. Clin Ther 2007; 29: 17-25. [PubMed: 17379044](Systematic review of literature on efficacy and safety of dutasteride and finasteride; similar rates of adverse events reported with each agent and were largely of sexual nature; no mention of hepatotoxicity or ALT elevations).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to either dutasteride or finasteride).
- De Nunzio C, Miano R, Trucchi A, Finazzi Agrò Tubaro A. Finasteride for prostatic disease: an updated and comprehensive review. Expert Opin Drug Metab Toxicol 2008; 4: 1561-8. [PubMed: 19040331](Review of pharmacology, clinical efficacy and safety of finasteride, no mention of liver injury or ALT elevations in the list of adverse events).
- Keam SJ, Scott LJ. Dutasteride: a review of its use in the management of prostate disorders. Drugs 2008; 68: 463-85. [PubMed: 18318566](Review of chemistry, pharmacology, clinical efficacy and safety of dutasteride; "Clinical laboratory measurements were unaffected by dutasteride therapy. Abnormalities occurred in similar proportions of dutasteride or placebo recipients").
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to dutasteride, finasteride or an agent used as therapy of benign prostatic hypertrophy).
- Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 2010; 146: 1141-50. [PubMed: 20956649](Systematic review of safety and efficacy of finasteride [1 and 5 mg daily] in 12 studies with 3927 patients with male pattern baldness, includes discussion of sexual adverse events only).
- Rossi A, Cantisani C, Scarnò M, Trucchia A, Fortuna MC, Calvieri S. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther 2011; 24: 455-61. [PubMed: 21910805](Among 118 men with male pattern baldness treated with finasteride [1 mg daily] for up to 10 years, side effects occurred in 5.9% and were mostly related to libido, erectile function and ejaculation; no mention of severe adverse events, ALT elevations or hepatotoxicity).
- Nickel JC, Gilling P, Tammela TL, Morrill B, Wilson TH, Rittmaster RS. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS). BJU Int 2011; 108: 388-94. [PubMed: 21631695](Among 1630 men with symptomatic prostatic hypertrophy treated with either finasteride or dutasteride for 12 months of whom 448 were continued on dutasteride in an open label study for up to 3 years, adverse events were similar and largely related to sexual desire and function; no mention of hepatotoxicity or ALT elevations).
- Kaplan SA, Chung DE, Lee RK, Scofield S, Te AE. A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int J Clin Pract 2012; 66: 1052-5. [PubMed: 23067029](Among 378 men with benign prostatic hyperplasia treated with dutasteride or finasteride for up to 5 years, side effects included loss of sexual desire and function; no mention of hepatotoxicity or ALT elevations).
- Park T, Choi JY. Efficacy and safety of dutasteride for the treatment of symptomatic benign prostatic hyperplasia (BPH): a systematic review and meta-analysis. World J Urol 2014; 32: 1093-105. [PubMed: 24500194](Systematic review of safety and efficacy of dutasteride in controlled trials of therapy of BPH discusses symptom adverse events, but does not mention ALT elevations or hepatotoxicity).
- Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol 2014; 70: 489-98. [PubMed: 24411083](Among 917 men with male pattern baldness treated with dutasteride, finasteride or placebo for 24 weeks, overall adverse events were similar and the "number of subjects with postbaseline laboratory and vital signs outside threshold values were similar across treatment groups").
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to dutasteride or finasteride).
- Yuan JQ, Mao C, Wong SY, Yang ZY, Fu XH, Dai XY, Tang JL. Comparative effectiveness and safety of monodrug therapies for lower urinary tract symptoms associated with benign prostatic hyperplasia: a network meta-analysis. Medicine (Baltimore) 2015; 94: e974. [PMC free article: PMC4504542] [PubMed: 26166130](In a metaanalysis of efficacy and safety of single agents, including finasteride and dutasteride, for relief of symptoms of benign prostatic hypertrophy, the authors conclude that "drug therapies were typically safe"; no mention of ALT elevations or hepatotoxicity).
- Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol 2016; 9: 56-62. [PMC free article: PMC5023004] [PubMed: 27672412](Systematic review of side effects of dutasteride and finasteride focusing on prostate cancer, psychiatric symptoms and sexual health in both men and women; no mention of ALT elevations or hepatotoxicity).
- Tsunemi Y, Irisawa R, Yoshiie H, Brotherton B, Ito H, Tsuboi R, Kawashima M, Manyak M; ARI114264 Study Group. Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J Dermatol 2016; 43: 1051-8. [PubMed: 26893187](Among 120 patients with alopecia treated with dutasteride for up to 1 year, adverse events inlcuded erectile dysfunction [12%] and decrease in libido [8%]; while increases in ALT levels were reported to occur, their frequency, severity and outcome were not reported).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia.[World J Urol. 2002]Review Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia.Bartsch G, Rittmaster RS, Klocker H. World J Urol. 2002 Apr; 19(6):413-25.
- Pharmacogenetic analysis of human steroid 5 alpha reductase type II: comparison of finasteride and dutasteride.[J Mol Endocrinol. 2005]Pharmacogenetic analysis of human steroid 5 alpha reductase type II: comparison of finasteride and dutasteride.Makridakis N, Reichardt JK. J Mol Endocrinol. 2005 Jun; 34(3):617-23.
- Review The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer.[J Urol. 2008]Review The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer.Tindall DJ, Rittmaster RS. J Urol. 2008 Apr; 179(4):1235-42. Epub 2008 Feb 20.
- Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5α-reductase inhibitors.[Prostate. 2013]Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5α-reductase inhibitors.Wu Y, Godoy A, Azzouni F, Wilton JH, Ip C, Mohler JL. Prostate. 2013 Sep; 73(13):1470-82. Epub 2013 Jun 27.
- The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men.[J Urol. 2008]The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men.Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, Swerdloff RS, Clark RV. J Urol. 2008 Jun; 179(6):2333-8. Epub 2008 Apr 18.
- Alpha Reductase Inhibitors - LiverToxAlpha Reductase Inhibitors - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...