NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Upadacitinib is an oral selective inhibitor of Janus associated kinase 1 (JAK-1) that is used in the therapy of moderate-to-severe rheumatoid arthritis. Upadacitinib has been associated with a low rate of serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent acute liver injury although it may pose a risk for reactivation of hepatitis B in susceptible patients.
Background
Upadacitinib (ue pad” a sye’ ti nib) is an orally available, small molecule inhibitor of Janus associated kinase (JAK), which is an essential component in the signal transducer and activator of transcription (STAT) pathway of immune activation and cytokine release. Inhibition of this pathway decreases T cell activation and proinflammatory cytokine release. Upadacitinib has a relative selectivity for JAK-1 and has been shown to improve symptoms and signs of active rheumatoid and psoriatic arthritis, ankylosing spondylitis, atopic dermatitis and ulcerative colitis. Upadacitinib was approved for use in 2019 in the United States as therapy for moderate or severely active rheumatoid arthritis in patients who have failed to respond or are intolerant of conventional disease modifying antirheumatic drugs (DMARDs), such as methotrexate and hydroxychloroquine or tumor necrosis factor (TNF) blockers. Since that time, the indications for upadacitinib have been expanded to include psoriatic arthritis, ankylosing spondylitis, ulcerative colitis and atopic dermatitis. The indications are generally limited to patients with moderately to severely active disease who have failed or were intolerant to therapy with systemic treatments such as TNF blockers. Upadacitinib is available in extended release tablets of 15, 30 and 45 mg under the brand name Rinvoq. The recommended maintenance dose is 15 mg once daily. Combination with other DMARDs or biologic agents is not recommended. Recommendations are limited to adults, except for atopic dermatitis (children 12 years or older). The regimen for ulcerative colitis includes an induction phase of 45 mg once daily for 8 weeks. Common side effects include mild-to-moderate symptoms of upper respiratory infection, cough, fever, fatigue, nausea, and laboratory abnormalities of lipids, liver and muscle enzymes. Uncommon, but potentially serious side effects include serious and opportunistic infections, reactivation of latent tuberculosis, herpes zoster, venous thromboses, gastrointestinal perforation, malignancy, major cardiovascular adverse events and embryo-fetal toxicity.
Hepatotoxicity
In the prelicensure clinical trials of upadacitinib in patients with rheumatoid arthritis, liver test abnormalities were frequent although usually mild. Some degree of ALT elevation arose in up to 11% of upadacitinib treated patients compared to 7% treated with placebo, but were above 3 times the upper limit of normal (ULN) in 2% or less. Furthermore, similar rates of ALT elevations arose in patients treated with methotrexate or biologic DMARDs. In these trials that enrolled over 3000 patients, there were no reports of clinically apparent liver injury, serious cases of liver injury or liver-related deaths. Similarly, other JAK inhibitors such as tofacitinib and baricitinib have been associated with frequent minor serum aminotransferase elevations during treatment, but episodes of clinically apparent liver injury have not been reported. Thus, this class of agents is suspected but not proven capable of causing liver injury.
In addition, long term treatment with upadacitinib and other Janus kinase inhibitors has been linked to rare instances of reactivation of hepatitis B that can be severe and has been linked to fatal outcomes. Reactivation can become clinically apparent after the JAK inhibitor is discontinued, when immune restoration results in an immunologic response to the heightened viral replication.
Likelihood score: D (possible, rare cause of clinically apparent liver injury including reactivation of hepatitis B in susceptible patients).
Mechanism of Injury
The cause of liver injury from upadacitinib is unknown, but with pattern of abnormalities suggests some degree of low level, direct hepatotoxicity. Thus, mean ALT levels rise slightly during the first 4 to 8 weeks of therapy (by approximately 5 U/L and usually remaining within the normal range) and return to baseline rapidly with stopping. In instances of more marked elevations, the abnormalities also resolve rapidly with discontinuation and may not recur with reexposure. Upadacitinib is metabolized in the liver via the cytochrome P450 system, largely CYP 3A4 (and by CYP 2D6 to a lesser extent) and is susceptible to drug-drug interactions with agents that inhibit or induce the CYP enzyme reactivity.
Reactivation of hepatitis B from JAK inhibitors, in contrast, is likely due to indirect effects of upadacitinib in causing immune suppression which may to lead to increased HBV replication which if followed by immune recovery, can result in acute liver injury.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. In patients with clinically apparent liver injury and jaundice, restarting therapy should be done with caution. Cross sensitivity to liver injury is uncommon among the tyrosine kinase inhibitors but there is no information or shared adverse event sensitivity among the small molecule JAK inhibitors. Tofacitinib and baricitinib have been linked to cases of reactivation of hepatitis B which can be severe. Importantly, patients who are to receive long term therapy with upadacitinib should be screened for hepatitis B (HBsAg and anti-HBc). Patients with HBsAg in serum should be treated prophylactically with an oral antiviral agent with activity against HBV such as entecavir or tenofovir. An alternative, which is applicable to those with anti-HBc without HBsAg, is to monitor patients carefully for HBV DNA levels to detect evidence of reactivation early and initiate appropriate therapy for HBV infection.
Drug Class: Antirheumatic Agents, Protein Kinase Inhibitors
Other Drugs in the Subclass, Janus Kinase Inhibitors for Rheumatoid Arthritis: Baricitinib, Tofacitinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Upadacitinib – Rinvoq®
DRUG CLASS
Antirheumatic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
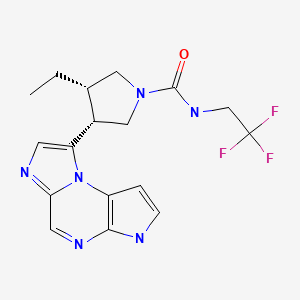
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Upadacitinib | 1310726-60-3 | C17-H19-F3-N6-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 August 2022
Abbreviations: DMARDs, disease modifying antirheumatic drugs; HBV, hepatitis B virus; JAK, Janus associated kinase; TNF, tumor necrosis factor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitors. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents, does not discuss upadacitinib).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/211675Orig1s000MedR.pdf. (FDA website with product labels and initial clinical review of the safety and efficacy of upadacitinib; states that upadacitinib recipients in a higher rate of ALT elevations than those on placebo [11.4% vs 7.2%] but rates were similar or lower in comparison to other disease modifying antirheumatic drugs [DMARDs], few episodes of aminotransferase elevations resulted in discontinuation, and no cases of liver injury with jaundice were reported, although one subject developed acute hepatitis B reactivation [ALT 1556 U/L, bilirubin 13.1 mg/dL, no information on HBV markers or outcome]). - Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, Zhou Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. [PubMed: 29908669](Among 661 adults with rheumatoid arthritis refractory to conventional DMARDs treated with upadacitinib [15 or 30 mg] or placebo daily for 12 weeks, response rates were higher with upadacitinib [64% and 66% vs 36%] while adverse events were slightly more frequent [57% and 54% vs 49%], although rates of “hepatic disorders” were similar in all 3 groups [2% and 3% vs 2%] and ALT elevations above 3 times ULN were rare [none and 0.9% vs 1.9%]).
- Bechman K, Subesinghe S, Norton S, Atzeni F, Galli M, Cope AP, Winthrop KL, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019;58(10):1755–1766. [PubMed: 30982883](Systematic review of controlled trials of JAK inhibitors for rheumatoid arthritis for serious infections identified 21 studies, providing rates per 100 patient-years of exposure for serious infections as 1.97 for tofacitinib, 3.16 baricitinib, and 3.02 upadacitinib vs 2.50 for placebo and for herpes zoster as 2.51 for tofacitinib, 3.16 baricitinib, 2.41 and upadacitinib vs 1.22 for placebo; no mention of HBV reactivation).
- Duggan S, Keam SJ. Upadacitinib: first approval. Drugs. 2019;79:1819–1828. [PubMed: 31642025](Review of the history of development, mechanism of action, pharmacokinetics, clinical efficacy, and safety in rheumatoid arthritis, as well as psoriatic arthritis, Crohn disease, ulcerative colitis, and atopic dermatitis, shortly after its approval for use in the United States; no mention of hepatotoxicity or ALT elevations).
- Upadacitinib (Rinvoq) - a new JAK inhibitor for rheumatoid arthritis. Med Lett Drugs Ther. 2019;61(1585):183–185. [PubMed: 31770358](Concise review of the mechanism of action, clinical efficacy, safety, and costs of upadacitinib shortly after its approval for use in rheumatoid arthritis in the US; no mention of hepatotoxicity or ALT elevations).
- van der Heijde D, Song IH, Pangan AL, Deodhar A, van den Bosch F, Maksymowych WP, Kim TH, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394(10214):2108–2117. [PubMed: 31732180](Among 187 adults with ankylosing spondylitis treated with upadacitinib vs placebo for 14 weeks, response rates were 52% vs 26% while adverse events were more frequent with upadacitinib [62% vs 55%], and while there were no serious infections or venous thrombosis, CPK elevations arose in 9% vs 2% and “hepatic disorders” in 5% vs 2%, but the ALT elevations were all asymptomatic, below 3 times ULN and transient, none requiring drug discontinuation).
- Drugs for psoriatic arthritis. Med Lett Drugs Ther. 2019;61(1588):203–210. [PubMed: 31999665](Concise review of the mechanism of action, relative clinical efficacy, safety and costs of drugs approved for use in psoriatic arthritis including NSAIDS, methotrexate, sulfasalazine, leflunomide, cyclosporine, apremilast, TNF inhibitors, ustekinumab, secukinumab, ixekizumab, abatacept, and the Jak inhibitor, tofacitinib; upadacitinib had been studied but had yet to be approved for this indication).
- van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MF, Chen S, Rischmueller M, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020;72:1607–1620. [PMC free article: PMC7589375] [PubMed: 32638504](Among 947 adults with rheumatoid arthritis and no or limited exposure to methotrexate treated with upadacitinib [15 or 30 mg daily] vs methotrexate [5-20 mg/kg weekly], response rates at 24 weeks were 48% and 50% vs 19% while adverse event rates were 64% and 71% vs 65%, and rates of “hepatic disorders” were equal across groups [6.0% and 4.5% vs 4.4%], while ALT elevations above 3 times ULN were more frequent with methotrexate [1.8% and 1.6% vs 4.4%]).
- Rubbert-Roth A, Enejosa J, Pangan AL, Haraoui B, Rischmueller M, Khan N, Zhang Y, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383:1511–1521. [PubMed: 33053283](Among 612 adults with rheumatoid arthritis refractory to DMARDs treated with upadacitinib vs abatacept for 24 weeks, upadacitinib was associated with greater decrease in symptom scores (-2.5 vs -2.0), higher rate of remission [30% vs 13%], but also higher rates of adverse events [69% vs 61%], serious adverse events [3.3% vs 1.6%], and ALT elevations above 5 times ULN [2.5% vs none], although none of the ALT elevations were accompanied by jaundice).
- Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, Zhang Y, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021;80(3):304–311. [PMC free article: PMC7892382] [PubMed: 33115760](Analysis of adverse events reported in 5 large clinical trials of upadacitinib for rheumatoid arthritis found that serious infections, herpes zoster, CPK elevations and gastrointestinal perforations were more frequent with upadacitinib than methotrexate while venous thromboses, malignancies, cardiovascular events, death and rates of ALT abnormalities were not more frequent).
- McInnes IB, Anderson JK, Magrey M, Merola JF, Liu Y, Kishimoto M, Jeka S, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227–1239. [PubMed: 33789011](Among 1704 adults with psoriatic arthritis refractory to conventional DMARDs therapy treated with upadacitinib [15 or 30 mg daily], placebo or adalimumab for 24 weeks, improvement in symptom scores was greater with upadacitinib [71% and 79% vs 36% with placebo and 65% adalimumab], while adverse event included similar rates of ALT elevations above 5 times ULN [0.9% and 1.2% vs 1.7% and 0.9%] and no patient developed ALT elevations accompanied by jaundice).
- Mysler E, Lizarraga A. Phase III trials of JAK1 selective inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2021;60 Suppl 2:ii17–ii23. [PMC free article: PMC8098104] [PubMed: 33950225](Review of efficacy results from 6 controlled trials of upadacitinib and 4 of filgotinib both of which are JAK-1 selective inhibitors reported 12-48 week response rates of 52-71% with upadacitinib vs 28-36% with placebo, and intermediate rates with methotrexate and adalimumab; no discussion of aminotransferase elevations or hepatotoxicity).
- Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology (Oxford). 2021;60 Suppl 2:ii24–ii30. [PMC free article: PMC8098103] [PubMed: 33950230](Review of the selective side effects of the JAK-1 inhibitors focusing upon infections [serious and non-serious and opportunistic including herpes zoster], venous thromboses, malignancy, blood disorders, changes in lipid levels, muscle enzymes, and hepatotoxicity states that elevations of aminotransferase levels occurs equally with JAK inhibitors, methotrexate and other biologic DMARDs and that no cases of ALT elevations with jaundice have been reported in the pre-registration clinical trials).
- Mease PJ, Lertratanakul A, Papp KA, van den Bosch FE, Tsuji S, Dokoupilova E, Keiserman MW, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-week data from the randomized controlled phase 3 SELECT-PsA 2 Study. Rheumatol Ther. 2021;8:903–919. [PMC free article: PMC8217417] [PubMed: 33913086](Among 641 adults with refractory psoriatic arthritis treated with upadacitinib [15 or 30 mg daily] or placebo for 24 weeks followed by upadacitinib in all for up to 56 weeks, response rates were similar with the two doses [60% and 59% at 56 weeks, vs ~20% at 24 weeks for placebo] while adverse events were slightly less common with the 15 mg dose, serious events in 14% vs 16%, herpes zoster in 4% vs 9%, “hepatic disorders” in 5% vs 18% although ALT elevations above 3 times ULN occurred in only 1% with both doses and no patient developed ALT elevations with jaundice).
- Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, Thaçi D, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. [PubMed: 34023008](Combined results of two large trials in a total of 1683 adolescents and adults with atopic dermatitis treated with upadacitinib [15 or 30 mg] or placebo for 16 weeks, found higher rates of response with upadacitinib [71% vs 15%] and slightly higher rate of total adverse events [64% vs 56%], but similar low rates of severe adverse events [2.3% vs 2.8%] and of “hepatic disorders” [1.6% vs 1.0%] with no instances of clinically apparent liver injury with jaundice).
- Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Werfel T, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. [PubMed: 34023009](Among 901 adolescents and adults with chronic atopic dermatitis treated with upadacitinib [15 or 30 mg] or placebo daily for 16 weeks, response rates were higher with upadacitinib [65% and 77% vs 26%] as were total adverse events [67% and 72% vs 63%], while severe adverse event rates were similar [1% and 1% vs s%], as were “hepatic disorders” [2% and 1% vs 2%] which were mostly transient, mild ALT elevations without symptoms or jaundice).
- Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, Prajapati VH, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. [PMC free article: PMC8340015] [PubMed: 34347860](Among 692 adults with atopic dermatitis treated for 24 weeks with upadacitinib [30 mg daily] or dupilumab [300 mg by injection every other week], response rates were higher with upadacitinib [71% vs 61%], but so were total adverse events [73% vs 63%], serious adverse events [2.9% vs 1.2%], and “hepatic disorders” [2.9% vs 1.2%] which were mostly transient, mild-to-moderate ALT elevations, although 2 patients on upadacitinib discontinued therapy early because of them).
- Drugs for rheumatoid arthritis. Med Lett Drugs Ther. 2021;63(1637):177–184. [PubMed: 35085210](Concise summary of the mechanism of action, clinical efficacy, toxicity and costs of drugs used to treat rheumatoid arthritis including baricitinib, tofacitinib and upadacitinib, common adverse events of which are gastrointestinal symptoms, nasopharyngitis and headache, while aminotransferase elevations can occur as well as dyslipidemia and cytopenias; rare adverse events may include major adverse cardiovascular events, thrombosis, malignancy and increase risk of death).
- Song YK, Song J, Kim K, Kwon JW. Potential adverse events reported with the Janus Kinase inhibitors approved for the treatment of rheumatoid arthritis using spontaneous reports and online patient reviews. Front Pharmacol. 2022;12:792877. [PMC free article: PMC8787189] [PubMed: 35087406](Analysis of spontaneous adverse event reports to the US and Korean Adverse Event Reporting Systems between 2013 and 2020 identified 21,708 reports on JAK inhibitors, predominantly tofacitinib [92], of which less than 1% were hepatobiliary).
- Burmester GR, Winthrop K, Blanco R, Nash P, Goupille P, Azevedo VF, Salvarani C, et al. Safety profile of upadacitinib up to 3 years in psoriatic arthritis: an integrated analysis of two pivotal phase 3 trials. Rheumatol Ther. 2022;9:521–539. [PMC free article: PMC8717827] [PubMed: 34970731](Pooled analysis of 2 controlled trials in patients with psoriatic arthritis treated with upadacitinib [15 or 30 mg once daily for up to 3 years], adalimumab [40 mg every other week] or placebo [for 6 months] found rates of adverse events similar in the four groups but higher rates of herpes zoster and CPK elevations with upadacitinib; ALT elevations above 5 times ULN arose in 1.3% on placebo, 1.9% on adalimumab and 1.2% and 1.7% on upadacitinib and no patient developed clinically apparent liver injury with jaundice).
- Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128. [PubMed: 35644166](Among 451 patients with ulcerative colitis who responded to an induction phase of upadacitinib [45 mg daily for 8 weeks] who were then treated with upadacitinib [15 or 30 mg] or placebo once daily for 52 weeks, no patient developed ALT elevations above 3 times upper limit or normal or required discontinuation of therapy because of hepatic events).
- van der Heijde D, Baraliakos X, Sieper J, Deodhar A, Inman RD, Kameda H, Zeng X, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis. 2022:annrheumdis-2022-222608. [PMC free article: PMC9606523] [PubMed: 35788492](Among 420 patients with ankylosing spondylitis treated with upadacitinib [15 mg] or placebo once daily for 14 weeks, response rates were higher with upadacitinib [45% vs 18%] as were adverse events [41% vs 37%], serious adverse events [2.8% vs 0.5%], serious infections [2.4% vs none] and ALT elevations [2.8% vs 1%] but there were no cases of clinically apparent liver injury with jaundice).
- Deodhar A, Van den Bosch F, Poddubnyy D, Maksymowych WP, van der Heijde D, Kim TH, Kishimoto M, et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10349):369–379. [PubMed: 35908570](Among 314 patients with active axial spondyloarthritis treated with upadacitinib [15 mg] or placebo once daily for 14 weeks, response rates were higher with upadacitinib [45% vs 23%] while adverse event rates were similar [48% vs 46%], including aminotransferase elevations [3% in both groups] and there were no cases of clinically apparent liver injury with jaundice).
- Upadacitinib (Rinvoq): a second JAK inhibitor for ulcerative colitis. Med Lett Drugs Ther. 2022;64(1658):142–144. [PubMed: 36094554](Concise summary of the mechanism of action, clinical efficacy, toxicity and costs of upadacitinib for ulcerative colitis, the second JAK inhibitor approved for this indication [the first being tofacitinib], mentions that it has been reported to cause liver enzyme elevations and rash).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Upadacitinib-Induced Hepatitis B Reactivation Leading to Liver Transplant.[ACG Case Rep J. 2024]Upadacitinib-Induced Hepatitis B Reactivation Leading to Liver Transplant.Khan SA, Zahid R, Amir M. ACG Case Rep J. 2024 Apr; 11(4):e01327. Epub 2024 Apr 5.
- Upadacitinib tartrate in rheumatoid arthritis.[Drugs Today (Barc). 2020]Upadacitinib tartrate in rheumatoid arthritis.Stamatis P, Bogdanos DP, Sakkas LI. Drugs Today (Barc). 2020 Nov; 56(11):723-732.
- Review A review of upadacitinib in rheumatoid arthritis.[Mod Rheumatol. 2020]Review A review of upadacitinib in rheumatoid arthritis.Tanaka Y. Mod Rheumatol. 2020 Sep; 30(5):779-787. Epub 2020 Jul 13.
- Review Upadacitinib for the Treatment of Rheumatoid Arthritis: An Extensive Review.[Ann Pharmacother. 2023]Review Upadacitinib for the Treatment of Rheumatoid Arthritis: An Extensive Review.Boyce EG, Rogan EL, C Lui M. Ann Pharmacother. 2023 Apr; 57(4):450-462. Epub 2022 Aug 2.
- Selectivity Profile of the Tyrosine Kinase 2 Inhibitor Deucravacitinib Compared with Janus Kinase 1/2/3 Inhibitors.[Dermatol Ther (Heidelb). 2021]Selectivity Profile of the Tyrosine Kinase 2 Inhibitor Deucravacitinib Compared with Janus Kinase 1/2/3 Inhibitors.Chimalakonda A, Burke J, Cheng L, Catlett I, Tagen M, Zhao Q, Patel A, Shen J, Girgis IG, Banerjee S, et al. Dermatol Ther (Heidelb). 2021 Oct; 11(5):1763-1776. Epub 2021 Aug 30.
- Upadacitinib - LiverToxUpadacitinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...