NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tofacitinib is an oral, small molecule inhibitor of Janus kinases that is used to treat moderate-to-severe rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease. Tofacitinib is associated with transient and usually mild elevations in serum aminotransferase levels during therapy, but has yet to be linked to cases of clinically apparent acute liver injury.
Background
Tofacitinib (tow" fa sye' ti nib) is an orally available, specific inhibitor of Janus-associated kinases (mainly JAK1 and JAK3) that is used to treat moderate-to-severe rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease. The Janus kinases are critical steps in immune activation as well as in hematopoiesis. The immunomodulatory effects of tofacitinib led to its evaluation in several autoimmune conditions including rheumatoid arthritis and psoriasis. In multiple, randomized controlled trials, tofacitinib was found to improve symptoms and signs of severe rheumatoid arthritis and psoriatic arthritis when used alone or in combination with other disease modifying antirheumatologic drugs (DMARDs). Tofacitinib was approved for use in rheumatoid arthritis in the United States in 2012. Subsequently, indications were expanded to psoriatic arthritis and inflammatory bowel disease. The Janus kinase inhibitors including tofacitinib are also being evaluated as therapy of the COVID-19, focusing upon alleviating the hyperinflammatory stages of advanced COVID-19 infection. At present, indications for tofacitinib are limited to moderate-to-severe rheumatoid arthritis and active psoriatic arthritis after failure or intolerance to methotrexate or other non-biological DMARDs, juvenile idiopathic arthritis with a polyarticular course, and moderately to severely active ulcerative colitis after failure of tumor necrosis factor (TNF) inhibitors. Tofacitinib is available in tablets of 5 and 10 mg under the brand name Xeljanz. The recommended dose is rheumatoid arthritis and psoriatic arthritis is 5 mg twice daily. For patients with active ulcerative colitis, an induction dose of 10 mg twice daily is recommended for the first three months. More recently, an extended release formulation of tofacitinib (Xeljanz XR, 11 and 22 mg tablets) that allows for once daily dosing has been made available. Common side effects of tofacitinib are neutropenia, headaches, diarrhea, fatigue, hypertension and symptoms of upper respiratory tract infection. Severe adverse events may include severe infections, reactivation of latent tuberculosis or herpes zoster, gastrointestinal perforation, venous and arterial thromboses, and de novo malignancies including Epstein-Barr virus related lymphoproliferative disorder.
Hepatotoxicity
In large registration clinical trials, serum aminotransferase elevations occurred in 28% to 34% of tofacitinib treated subjects compared to 25% in comparator arms and 10% in placebo recipients. These elevations were typically mild and transient, but values above 3 times the upper limit of normal (ULN) occurred in 1% to 2% of patients on tofacitinib compared to less than 1% on placebo. The elevations occasionally led to early discontinuations, but more often resolved even without dose adjustment. In prelicensure studies, there were no instances of clinically apparent liver injury attributed to tofacitinib. Since approval and more wide scale availability of tofacitinib, there have been no published reports of hepatotoxicity associated with its use but a proportion of patients do develop serum aminotransferase elevations which in some cases leads to drug discontinuation. While other Janus kinase inhibitors such as ruxolitinib have been associated with episodes of reactivation of hepatitis B, spontaneous reports of clinically apparent reactivation of hepatitis during tofacitinib therapy have not been reported. On the other hand, retrospective studies on patients with HBsAg and inactive liver disease who were treated with tofacitinib have been reported to develop rising levels of HBV DNA and modest elevations in serum aminotransferase levels without symptoms. In contrast, studies of patients with anti-HBc without HBsAg in serum have shown no evidence of HBV DNA rises and appearance of HBsAg. Thus, reactivation of hepatitis B during therapy can occur, although it is generally mild and self-limited in course. Whether reactivation of hepatitis B can arise after therapy of susceptible patients with tofacitinib for severe COVID-19 pneumonia is unknown, but there have been no such reports to date.
Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury with the potential to cause reactivation of hepatitis B).
Mechanism of Injury
The causes of serum enzyme elevations during tofacitinib therapy are not known. Tofacitinib is metabolized in the liver largely through the CYP 3A4 pathway and liver injury may be related to production of a toxic or immunogenic intermediate. Because it is a substrate for CYP 3A4, tofacitinib is susceptible to drug-drug interactions with agents that inhibit or induce this specific hepatic microsomal activity. Tofacitinib is a potent immunomodulatory agent and appears to be capable of causing reactivation of hepatitis B.
Outcome and Management
Monitoring of serum aminotransferase levels is recommended for patients starting tofacitinib. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) or any elevations accompanied by jaundice or symptoms should lead to dose reduction or temporary cessation. There are no data to suggest a cross reactivity in risk for hepatic injury between tofacitinib and other kinase inhibitors or biologic or nonbiologic DMARDs. Because tofacitinib is capable of inducing reactivation of hepatitis B, patients starting long term therapy with tofacitinib should be screened for HBsAg and anti-HBc. Patients with preexisting HBsAg in serum should undergo evaluation and prophylaxis against reactivation of HBV using potent oral antiviral agents, such as tenofovir or entecavir. Those with anti-HBc without HBsAg or HBV DNA should be monitored for evidence of infection and treated if there is de novo appearance of HBsAg or HBV DNA.
Drug Class: Antirheumatic Agents, Protein Kinase Inhibitors, COVID-19 Drugs
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tofacitinib – Xeljanz®
DRUG CLASS
Antirheumatic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ruxolitinib | 941678-49-5 | C17-H18-N6 |
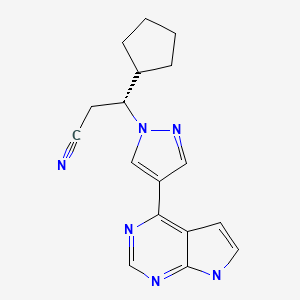
|
| Tofacitinib | 477600-75-2 | C16-H20-N6-O |
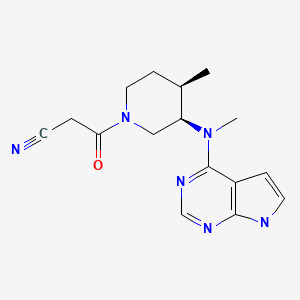
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 August 2022
- Zimmerman HJ. Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of protein kinase inhibitors such as tofacitinib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents discusses several kinase inhibitors including imatinib, gefitinib, erlotinib and crizotinib, but not tofacitinib).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics; tofacitinib is not discussed specifically).
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. [PubMed: 19565475](Among 264 patients with rheumatoid arthritis treated with tofacitinib [5, 15 or 30 mg] or placebo twice daily for 6 weeks, symptomatic improvements occurred within 1 week of starting tofacitinib and the most common adverse events were headache and nausea; no mention of ALT elevations or hepatotoxicity).
- Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH., Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63:1150–8. [PubMed: 21584942](Among 140 patients with rheumatoid arthritis receiving methotrexate treated with tofacitinib [1, 3, 5 or 10 mg] or placebo twice daily for 12 weeks, ALT elevations occurred in 19% of patients on tofacitinib [vs 4% on placebo], but levels were above 3 times ULN in only 3% and no patient developed clinically apparent liver injury).
- Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, Gupta P, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–77. [PubMed: 22924949](Among 197 patients with plaque psoriasis treated with tofacitinib [2, 5 or 15 mg] or placebo twice daily for 12 weeks, adverse event rates were similar with tofacitinib and placebo; one patient [0.7%] discontinued therapy because of ALT elevations).
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81. [PubMed: 22006202](Among 507 patients with rheumatoid arthritis with an inadequate response to methotrexate treated with tofacitinib in several doses or placebo for 12 weeks, clinical responses were better with tofacitinib and common adverse events included diarrhea, headache and infections; ALT elevations above 3 times ULN occurring most frequently with the higher doses of tofacitinib, leading to 3 patients discontinuing therapy early).
- Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–29. [PubMed: 21952978](Among 384 patients with rheumatoid arthritis treated with tofacitinib in varying doses or adalimumab or placebo for 12 weeks, ALT elevations occurred in 17% on tofacitinib vs 20% on placebo and were above 3 times ULN in 1% vs 0%).
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, et al. ORAL Standard Investigators. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. [PubMed: 22873531](Among 717 patients with rheumatoid arthritis on methotrexate treated with tofacitinib [5 or 10 mg] or adalimumab or placebo for 52 weeks, clinical response rates were higher for tofacitinib [52-53%] than placebo [28%] and adverse events were more common [with two cases of pulmonary tuberculosis]; ALT elevations during the first 3 months occurred in 26% on tofacitinib vs 17% on placebo).
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, et al. ORAL Solo Investigators. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. [PubMed: 22873530](Among 611 patients with rheumatoid arthritis treated with tofacitinib [5 or 10 mg] or placebo twice daily for 3 months, clinical response rates were higher with tofacitinib, and adverse events included headache, infections, neutropenia and increases in serum cholesterol; ALT elevations above 3 times ULN occurred during the first 3 months in <1% of both groups).
- Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–61. [PubMed: 24026258](Among 795 patients with rheumatoid arthritis [and an inadequate response to DMARDs] treated with tofacitinib [5 or 10 mg] or placebo twice daily for 3 months with extension of active treatment for 1 year, 3 tofacitinib treated patients stopped therapy early because of ALT or AST elevations, but there were no reports of clinically apparent liver injury).
- Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, et al. ORAL Step investigators. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60. [PubMed: 23294500](Among 399 patients with rheumatoid arthritis [and an inadequate response to antitumor necrosis factor agents] treated with tofacitinib [5 or 10 mg] or placebo twice daily for 3 months, ALT elevations occurred in 17% on tofacitinib vs 13% on placebo, but were above 3 times ULN in 0.8% vs 0% and no patient developed clinically apparent liver injury).
- Tofacitinib (Xeljanz) for rheumatoid arthritis. Med Lett Drugs Ther. 2013;55(1407):1–3. [PubMed: 23288133](Concise review of the mechanism of action, pharmacology, efficacy, safety and costs of tofacitinib shortly after its approval for in the US, mentions that elevations in ALT and AST have occurred with therapy and that “liver enzymes should be monitored regularly”).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; tofacitinib is not discussed).
- Spraggs CF, Xu CF, Hunt CM. Genetic characterization to improve interpretation and clinical management of hepatotoxicity caused by tyrosine kinase inhibitors. Pharmacogenomics. 2013;14:541–54. [PubMed: 23556451](Review of genetic associations of serum ALT and bilirubin elevations during therapy with tyrosine kinase inhibitors focusing on lapatinib and pazopanib; tofacitinib is not mentioned).
- Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, Nduaka CI, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41:837–52. [PubMed: 24692527](Among 4102 patients with rheumatoid arthritis treated with tofacitinib for up to 5 years in 2 open label studies following participation in placebo controlled trials, 21% of patients discontinued treatment and serious adverse events occurred in 15%, with ALT elevations above 3 times ULN in 48 patients [1.2%], 22 discontinuing treatment for this reason, and one patient having persistent ALT elevations and later diagnosis of autoimmune hepatitis requiring corticosteroid therapy).
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, Langley R, et al. OPT Pivotal 1 and OPT Pivotal 2 investigators. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–61. [PubMed: 26149717](Among 1861 patients with plaque psoriasis treated with tofacitinib [5 or 10 mg] or placebo twice daily for 16 weeks, higher rates of improvement occurred with tofacitinib [42-59%] than placebo [9-11%], while adverse event rates were similar overall; ALT elevations above 3 times ULN occurred in 0.6% vs 0.2% of patients).
- Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25:514–21. [PMC free article: PMC4819568] [PubMed: 25496464](Among 317 Japanese patients with rheumatoid arthritis treated with tofacitinib [1, 3, 5, 10 or 15 mg] or placebo twice daily for 12 weeks, ALT elevations above 3 times ULN occurred in 0-1.9% of tofacitinib vs 3.8% of placebo recipients and led to early discontinuation of therapy in one tofacitinib treated subject).
- Cohen SB, Koenig A, Wang L, Kwok K, Mebus CA, Riese RJ, Fleischmann R. Efficacy and safety of tofacitinib in US and non-US rheumatoid arthritis patients: pooled analyses of phase II and III. Clin Exp Rheumatol. 2016;34:32–6. [PubMed: 26575982](Pooled data on side effects from more than 3000 patients with rheumatoid arthritis focused largely on respiratory infections, diarrhea and edema; there were no cases of tuberculosis, lymphoma or serious herpes zoster infections; no mention of hepatotoxicity or reactivation of hepatitis B).
- Charles-Schoeman C, Burmester G, Nash P, Zerbini CA, Soma K, Kwok K, Hendrikx T, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2016;75:1293–301. [PMC free article: PMC4941182] [PubMed: 26275429](Among 3517 patients with rheumatoid arthritis participating in 9 placebo controlled trials of tofacitinib, response as well as serious adverse event rates were similar among those who had never received and those who had an inadequate response to a previous course of a biological DMARD; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 cases were attributed to drugs for rheumatoid arthritis: all 5 due to leflunomide and none to tofacitinib).
- Papp KA, Krueger JG, Feldman SR, Langley RG, Thaci D, Torii H, Tyring S, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016;74:841–50. [PubMed: 26899199](Among 1861 patients with plaque psoriasis treated with tofacitinib or placebo for 16 weeks who were then all treated for up to 24 months, initial responses were maintained for the duration of treatment and risk for adverse events did not increase over time, although there were eventually 67 cases of herpes zoster and 5 deaths, none of which were considered related to tofacitinib; no mention of hepatotoxicity or reactivation of hepatitis B).
- Chen MH, Chen MH, Liu CY, Tsai CY, Huang DF, Lin HY, Lee MH, et al. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J Infect Dis. 2017;215:566–573. [PubMed: 28011918](Among 123 HBsAg positive patients with rheumatoid arthritis not given antiviral prophylaxis, 30 [24%] developed HBV reactivation, including 18 of 51 on corticosteroids and 12 of 36 [33%] on biologic agents such as anti-TNF (8 of 26: 31%]] and rituximab [3 of 6: 50%]).
- Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, DeMasi R, et al. ORAL Strategy investigators. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390(10093):457–68. [PubMed: 28629665](Among 1146 patients with rheumatoid arthritis treated with tofacitinib alone or with methotrexate or adalimumab for 1 year, response rates were slightly higher with addition of methotrexate [46%] and adalimumab [44%] compared to tofacitinib alone, as were ALT elevations above 3 times ULN [4% and 4% vs 2%], and 2 patients developed "drug-induced liver injury").
- Zhang J, Tsai TF, Lee MG, Zheng M, Wang G, Jin H, Gu J, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: A Phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88:36–45. [PubMed: 28558978](Among 266 Asian patients with plaque psoriasis treated with tofacitinib or placebo for 16 weeks and then "advanced" to tofacitinib, only 3 patients had ALT elevations above 3 times ULN and none developed symptoms or jaundice).
- Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, Panaccione R, et al. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–59. [PMC free article: PMC5532457] [PubMed: 28209624](Among 280 patients enrolled in 8 week placebo controlled trials of 2 doses of tofacitinib for Crohn’s disease, 180 enrolled in a maintenance trial for up to 24 weeks; total and severe adverse event rates were similar in all groups; no mention of ALT elevations or hepatotoxicity or of reactivation of hepatitis B).
- Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, Winthrop KL, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5+ years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–62. [PMC free article: PMC5530353] [PubMed: 28143815](Analysis of long term safety of tofacitinib based upon pooled data on 6194 patients with rheumatoid arthritis found incidence rates of herpes zoster to be 3.9 per 100 patient-years, 2.7 for serious infections and 0.2 for tuberculosis, but there were no instances of reactivation of hepatitis B mentioned).
- Cohen S, Curtis JR, DeMasi R, Chen Y, Fan H, Soonasra A, Fleischmann R. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther. 2018;5:283–91. [PMC free article: PMC5935628] [PubMed: 29470834](Postmarketing reports of adverse events over a 3 year period identified 4352 serious adverse events, the most frequent of which were infections [n=827, 2.6 per 100 patient-yrs] and hepatobiliary disorders were rare [n=41, 0.12 per 100 patient-yrs], but no details provided).
- Valenzuela F, Korman NJ, Bissonnette R, Bakos N, Tsai TF, Harper MK, Ports WC, et al. Tofacitinib in patients with moderate to severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol. 2018;179(4):853–62. [PubMed: 29782642](Among 2867 patients treated with tofacitinib for a median of 3 years, adverse events arose in 2366 [83%] patients, including 176 [6%] with herpes zoster and 1 with tuberculosis, but none with reactivation of hepatitis B or clinically apparent liver injury with jaundice).
- Chen YM, Huang WN, Wu YD, Lin CT, Chen YH, Chen DY, Hsieh TY. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis. 2018;77:780–2. [PubMed: 28663308](Among 116 Taiwanese patients with rheumatoid arthritis treated with tofacitinib at a single center over a 2 year period, 81 had serologic evidence of ongoing or previous HBV infection; 2 of the 6 HBsAg positive [2 received prophylaxis] but none of the 75 with anti-HBc without HBsAg developed evidence of reactivation [de novo appearance of HBV DNA], which triggered rapid initiation of therapy with entecavir).
- Zhang Z, Deng W, Wu Q, Sun L. Tuberculosis, hepatitis B and herpes zoster in tofacitinib-treated patients with rheumatoid arthritis. Immunotherapy. 2019;11:321–33. [PubMed: 30630365](Systematic review of the literature of rates of tuberculosis, HBV and herpes zoster in patients treated in clinical trials of long term tofacitinib [19,406 patient years] found that most studies excluded patients with chronic hepatitis B or C, but studies from Taiwan [Chen et al, 2018] reported HBV reactivation in 2 of 4 subjects with HBsAg [not given prophylaxis] and 1.7% of patients overall, while rates of tuberculosis on long-term tofacitinib were 0.17 and herpes zoster were 3.9 per 100 patient-years).
- Drugs for psoriatic arthritis. Med Lett Drugs Ther. 2019;61(1588):203–10. [PubMed: 31999665](Concise review of the mechanism of action, relative clinical efficacy, safety and costs of drugs in use for therapy of psoriatic arthritis including NSAIDS, apremilast, TNF inhibitors, ustekinumab, secukinumab, ixekizumab, abatacept, and tofacitinib mentions that tofacitinib can cause aminotransferase elevations and has been linked to venous thromboses that can be severe and even fatal).
- Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, Winthrop KL, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. [PMC free article: PMC7722371] [PubMed: 33127856](Among 7061 adults with rheumatoid arthritis treated with tofacitinib for a median of 3.1 years, 782 [11.1%] developed herpes zoster, 576 [8.2%] had serious infections, 38 [05%] developed active tuberculosis and venous thrombotic events in 59 [1%]; no mention of ALT elevations or hepatoxicity).
- Palasik BN, Wang H. Tofacitinib, the first oral janus kinase inhibitor approved for adult ulcerative colitis. J Pharm Pract. 2021;34(6):913–921. [PubMed: 32873116](Summary of the structure, mechanism of action, pharmacology, clinical efficacy and safety of tofacitinib).
- Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, Bader G, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–1601.e3. [PMC free article: PMC7779667] [PubMed: 32629130](Among 260 patients with ulcerative colitis or Crohn’s disease treated with tofacitinib and followed for a median of 6 months and monitored for side effects, 15% had at least one adverse event included 13.5% with infection; 5 had herpes zoster and 2 had venous thrombotic events; no mention of ALT elevations or hepatotoxicity).
- Nash P, Coates LC, Kivitz AJ, Mease PJ, Gladman DD, Covarrubias-Cobos JA, FitzGerald O, et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: interim analysis of OPAL Balance, an open-label, long-term extension study. Rheumatol Ther. 2020;7:553–80. [PMC free article: PMC7410915] [PubMed: 32506317](Among 686 patients with psoriatic arthritis treated with tofacitinib for up to 3 years, herpes zoster developed in 19 patients [2.8%], serious infections in 11 [1.6%] and ALT elevations in 27 [4%], but no patient developed clinically apparent liver injury with jaundice).
- Serling-Boyd N, Mohareb AM, Kim AY, Hyle EP, Wallace ZS. The use of tocilizumab and tofacitinib in patients with resolved hepatitis B infection: a case series. Ann Rheum Dis. 2021;80(2):274–6. [PMC free article: PMC7855328] [PubMed: 32732241](Among 20 US patients with rheumatoid arthritis who were positive for anti-HBc without HBsAg in serum and who were treated with tocilizumab [n=16] and/or tofacitinib [n=8], none developed evidence of reactivation of hepatitis B, however routine monitoring for HBV DNA was not done).
- Watanabe R, Hashimoto M, Morinobu A. Correspondence on 'The use of tocilizumab and tofacitinib in patients with resolved hepatitis B infection: a case series'. 2020 Nov 3: annrheumdis-2020-219270. [PubMed: 33144301](Letter in response to Serling-Boyd [2020] suggesting use of routine screening for hepatitis B markers in patients receiving advanced immunotherapies such as tocilizumab and tofacitinib).
- Jacobs J, Clark-Snustad K, Lee S. Case report of a SARS-CoV-2 infection in a patient with ulcerative colitis on tofacitinib. Inflamm Bowel Dis. 2020;26:e64. [PMC free article: PMC7197538] [PubMed: 32342098](33 year old woman with ulcerative colitis had a mildly symptomatic, self-limited case of COVID-19 and no worsening of her gastrointestinal disease despite continuing tofacitinib during the infection).
- Peterson D, Damsky W, King B. The use of Janus kinase inhibitors in the time of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Am Acad Dermatol. 2020;82:e223–e226. [PMC free article: PMC7144601] [PubMed: 32278797](Letter expressing caution about use of Janus kinase inhibitors during COVID 19 infection based upon their mechanism of action in blocking intracellular signaling pathways mediated by Janus kinases which might block normal immunologic function, but also mentioning 3 patients with alopecia areata who developed COVID-19 infection while on an inhibitor and had a relative mild, normal course of infection).
- Satarker S, Tom AA, Shaji RA, Alosious A, Luvis M, Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad Med. 2021;133(5):489–507. [PMC free article: PMC7784782] [PubMed: 33245005](Review of the mechanism of action and pleomorphic activities of Janus kinase inhibitors and their possible role in treating severe COVID-19 infection which is often accompanied by high levels of proinflammatory cytokines [cytokine storm], with listing of ongoing trials including several of tofacitinib).
- Wong HS, Guo CL, Lin GH, Lee KY, Okada Y, Chang WC. Transcriptome network analyses in human coronavirus infections suggest a rational use of immunomodulatory drugs for COVID-19 therapy. Genomics. 2021;113:564–75. [PMC free article: PMC7817445] [PubMed: 33482326](Analysis of the temporal pattern of upregulation of genes in cells infected with SARS-CoV-2 suggest that medications most likely to affect these changes include among others dexamethasone, baricitinib, tofacitinib, sarilumab, ritonavir, naproxen, avelumab, durvalumab and atezolizumab).
- Gatti M, Turrini E, Raschi E, Sestili P, Fimognari C. Janus kinase inhibitors and coronavirus disease (COVID)-19: rationale, clinical evidence and safety issues. Pharmaceuticals (Basel). 2021;14:738. [PMC free article: PMC8401109] [PubMed: 34451835](Review of the theoretic basis for use of JAK inhibitors in COVID-19 disease, clinical efficacy and safety of baricitinib and ruxolitinib mentions rare instances of ALT elevations and one case of drug-induced liver injury).
- Raghuvanshi R, Bharate SB. Recent developments in the use of kinase inhibitors for management of viral infections. J Med Chem. 2022;65:893–921. [PubMed: 33539089](Review of the kinase inhibitors approved for use in the US, their mechanisms of action and possible role in inhibiting viral replication).
- Maslennikov R, Ivashkin V, Vasilieva E, Chipurik M, Semikova P, Semenets V, Russkova T, et al. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm Pharmacol Ther. 2021;69:102039. [PMC free article: PMC8137353] [PubMed: 34023513](Among 32 patients with COVID-19 and CRP elevations treated with tofacitinib for 5 days, the mortality rate was 17% and adverse events included ALT elevations in 59%, above 3 times ULN in 25% and above 10 times ULN in 6%).
- Huang JX, Zhang LJ. Successful treatment of tofacitinib in a case with rheumatoid arthritis who experienced hepatitis B virus reactivation induced by tocilizumab and recovered from entecavir rescue therapy. Arch Rheumatol. 2020;36:458–460. [PMC free article: PMC8612488] [PubMed: 34870178](77 year old man with rheumatoid arthritis and HBsAg with normal ALT and low levels of HBV DNA was treated with tocilizumab and developed rises in HBV DNA [10 million IU/mL] and ALT [221 U/L], which responded to entecavir and did not recur when tofacitinib was started).
- Wang ST, Tseng CW, Hsu CW, Tung CH, Huang KY, Lu MC, Lai NS. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib. Int J Rheum Dis. 2021;24:1362–1369. [PubMed: 34506078](Among 98 patients with rheumatoid arthritis with HBsAg in serum [n=8] or anti-HBc without HBsAg [n=64] treated with tofacitinib at a single Taiwanese hospital between 2015 and 2020, two with HBsAg and two without developed virologic evidence of HBV reactivation [after 11 to 57 months], but all recovered spontaneously [without antiviral therapy], none developed symptoms or jaundice and only one had transient ALT elevations [peak 104 U/L]).
- Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, Lens S, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89–94. [PMC free article: PMC7537127] [PubMed: 32969557](Among 61 patients with severe COVID-19 who received immunomodulatory therapy [corticosteroids in 100%, tocilizumab in 72%, baricitinib in 3%] and were HBsAg negative but anti-HBc positive, 38 were given antiviral prophylaxis with entecavir and 23 were not, but none developed HBV reactivation, although 2 [no prophylaxis, anti-HBs negative] developed transient low levels of HBV DNA [<10 IU/mL] without aminotransferase elevations).
- Mazza S, Soro S, Verga MC, Elvo B, Ferretti F, Cereatti F, Drago A, et al. Liver-side of inflammatory bowel diseases: Hepatobiliary and drug-induced disorders. World J Hepatol. 2021;13:1828–1849. [PMC free article: PMC8727201] [PubMed: 35069993](Review of the hepatic manifestations and complications of inflammatory bowel disease including sclerosing cholangitis, gallstones, nonalcoholic hepatitis, portal vein thrombosis and drug induced liver injury caused by sulfasalazine, azathioprine, thioguanine, methotrexate, TNF inhibitors and tofacitinib mentions that no case of clinical apparent liver injury has been reported with tofacitinib therapy).
- Hoisnard L, Lebrun-Vignes B, Maury S, Mahevas M, El Karoui K, Roy L, Zarour A, et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. 2022;12:7140. [PMC free article: PMC9065106] [PubMed: 35504889](Among 126,815 individual case safety reports on ruxolitinib, tofacitinib and baricitinib made to the WHO pharmacovigilance registry, infections were most frequent including viral, fungal, bacterial and mycobacterial complications while there was no increase in hepatobiliary reports).
- Mohareb AM, Patel NJ, Fu X, Kim AY, Wallace ZS, Hyle EP. Screening for hepatitis B virus prior to initiating tocilizumab and tofacitinib in patients with rheumatic diseases: a cross-sectional study. J Rheumatol. 2022;49:104–109. [PMC free article: PMC8724454] [PubMed: 34334359](Survey of 678 patients starting tocilizumab and 307 tofacitinib therapy found that only 29% and 24% were tested for HBsAg, anti-HBc and anti-HBs before starting therapy [despite CDC recommendations] and an average of 30% had inappropriate tests done).
- Avni-Biron I, Bar-Gil Shitrit A, Koslowsky B, Levartovsky A, Kopylov U, Weisshof R, Aviv Cohen N, et al. Short-term effectiveness and safety of tofacitinib in ulcerative colitis - real world data from tertiary medical centers in Israel. Dig Liver Dis. 2022;54:192–197. [PubMed: 34887214](Among 73 patients with ulcerative colitis treated with tofacitinib in general medical practice in Israel, the response rate was 65% and 17 patients had adverse events including 2 with herpes zoster [2.7%], while there were no reports of elevations in liver enzymes).
- Kostik MM, Raupov RK, Suspitsin EN, Isupova EA, Gaidar EV, Gabrusskaya TV, Kaneva MA, et al. The safety and efficacy of tofacitinib in 24 cases of pediatric rheumatic diseases: single centre experience. Front Pediatr. 2022;10:820586. [PMC free article: PMC8861449] [PubMed: 35211430](Among 24 children with juvenile rheumatoid arthritis, autoinflammatory diseases or dermatomyositis treated with tofacitinib for 6 months or more, complete responses occurred in half and adverse events were uncommon, 2 children had liver enzyme elevations that resolved without drug discontinuation).
- D'Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, et al. Impaired coagulation, liver dysfunction and COVID-19: discovering an intriguing relationship. World J Gastroenterol. 2022;28:1102–1112. [PMC free article: PMC8985482] [PubMed: 35431501](Review of the hepatic manifestations of SARS-CoV-2 infection including direct effects of the viral infection, and secondary effects from cytokines as well as drug induced liver injury, mentioning the possible contribution of tofacitinib).
- Yip TC, Gill M, Wong GL, Liu K. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol Int. 2022;16:257–268. [PMC free article: PMC8889512] [PubMed: 35235148](Review of published data on reactivation of hepatitis B caused by treatment of COVID focusing upon corticosteroids, baricitinib, tofacitinib, sarilumab and tocilizumab, which are capable of inducing reactivation when used in rheumatic disease, but have not been reported with their use for COVID-19 perhaps due to duration of therapy or the heightened immune activity of the viral infection).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Baricitinib.[LiverTox: Clinical and Researc...]Review Baricitinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Frequency and Duration of Early Non-serious Adverse Events in Patients with Rheumatoid Arthritis and Psoriatic Arthritis Treated with Tofacitinib.[Rheumatol Ther. 2022]Frequency and Duration of Early Non-serious Adverse Events in Patients with Rheumatoid Arthritis and Psoriatic Arthritis Treated with Tofacitinib.Dikranian A, Gold D, Bessette L, Nash P, Azevedo VF, Wang L, Woolcott J, Shapiro AB, Szumski A, Fleishaker D, et al. Rheumatol Ther. 2022 Apr; 9(2):411-433. Epub 2021 Dec 17.
- Tofacitinib Use in Adults with Chronic Inflammatory Disease During the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic: What Is Known So Far?[Curr Ther Res Clin Exp. 2021]Tofacitinib Use in Adults with Chronic Inflammatory Disease During the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic: What Is Known So Far?Howland S, Deuring JJ, Zhou X, Chen Y, Mota LM, Ungaro RC. Curr Ther Res Clin Exp. 2021; 95:100639. Epub 2021 Jul 25.
- Review Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis.[Ann Pharmacother. 2013]Review Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis.Vyas D, O'Dell KM, Bandy JL, Boyce EG. Ann Pharmacother. 2013 Nov; 47(11):1524-31.
- Review Abrocitinib.[LiverTox: Clinical and Researc...]Review Abrocitinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Tofacitinib - LiverToxTofacitinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...