NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Trimetrexate is a parenterally administered folate antagonist that is used as a second line therapy for severe Pneumocystis jirovecii (previously carinii) pneumonia. Trimetrexate therapy has been associated with transient, mild serum enzyme elevations during therapy, but has not been convincingly linked to instances of acute, clinically apparent liver injury.
Background
Trimetrexate (trye" me trex' ate) is a folic acid analogue which acts as an antagonist inhibiting the enzymes involved in folate dependent synthetic pathways such as thymidine synthase, dihydrofolate reductase and glycinamide ribonucleotide formyltransferase. Inhibition of these enzymes leads to decrease in intracellular thymidine and purine which interferes with RNA and DNA synthesis and leads to apoptotic cell death in rapidly dividing cells. Trimetrexate is a nonclassical folate antagonist and, unlike methotrexate, is lipid soluble which leads to different pharmacokinetics and tissue distribution. Trimetrexate was developed as an antineoplastic agent and showed some activity against breast, lung and head and neck cancer, but had difficult toxicities and never received approval for these uses in the United States. Trimetrexate is also taken up by pathogens including Pneumocystis jirovecii where it blocks the folate dependent synthetic pathways and causes their death. At the same time, folate being water soluble can partially block the systemic effects of trimetrexate, but is not taken up by the pathogens. For this reason, high doses of trimetrexate combined with leucovorin “rescue” were evaluated as therapy of Pneumocystis jirovecii in patients who were resistant or intolerant of first line anti-Pneumocystis therapies. Trimetrexate was approved for use as a second line therapy of moderate-to-severe Pneumocystis jirovecii pneumonia in immunodeficient patients in the United States in 1993. It remains available as a second line drug for this indication, but has not been widely used. Trimetrexate is available in single use vials of 25 or 200 mg as a powder for reconstitution under the brand name Neutrexin. The recommended dose is 45 mg/m2 once daily for 21 days concurrent with leucovorin, orally or intravenously, four times daily for 24 days. When given with leucovorin, trimetrexate is well tolerated, but can cause many of the common side effects of other folate antagonists such as fatigue, nausea, vomiting, anorexia, diarrhea, alopecia, anemia, neutropenia and rash. Uncommon, but potentially serious adverse events include febrile neutropenia, infections, dehydration, renal failure, arrhythmias and peripheral neuropathy.
Hepatotoxicity
When given without leucovorin protection, trimetrexate therapy is associated with a moderate rate of serum enzyme elevations, serum ALT or AST elevations above 5 times ULN in up to 20% of patients. When given with leucovorin, however, trimetrexate has fewer side effects although serum enzyme elevations can still occur. In clinical trials in patients with HIV infection and Pneumocystis jirovecii pneumonia, ALT elevations above 5 times ULN occurred in 1% to 8% of patients, but were usually no more frequent than with standard therapy using trimethoprim with sulfamethoxazole. The elevations were typically transient, without accompanying symptoms or jaundice and resolved or improved despite continuation of therapy. No instances of clinically apparent acute liver injury attributed to trimetrexate have been reported in the literature. In addition, trimetrexate has not been linked to sinusoidal obstruction syndrome or to reactivation of hepatitis B. Nevertheless, trimetrexate probably has hepatotoxic potential, but because it has limited use, is given for short periods of time and is administered with leucovorin, it has not been convincingly linked to cases of clinically apparent liver injury with jaundice.
Likelihood score: E* (unproven but suspected cause of liver injury).
Mechanism of Injury
The cause of the serum enzyme elevations that may occur during trimetrexate use is probably direct hepatotoxicity from the folate antagonism. Its hepatic metabolism has not been well defined.
Outcome and Management
Trimetrexate therapy has been linked to serum enzyme elevations in a small-to-moderate proportion of patients, but these elevations rarely require dose modifications or early discontinuation of treatment. The elevations often decrease even with continuation of treatment. There have been no instances of idiosyncratic liver injury, acute liver failure, chronic hepatitis or vanishing bile duct syndrome linked to trimetrexate therapy.
Drug Class: Antineoplastic Agents, Antifolate Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trimetrexate – Neutrexin®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Trimetrexate | 52128-35-5 | C19-H23-N5-O3 |
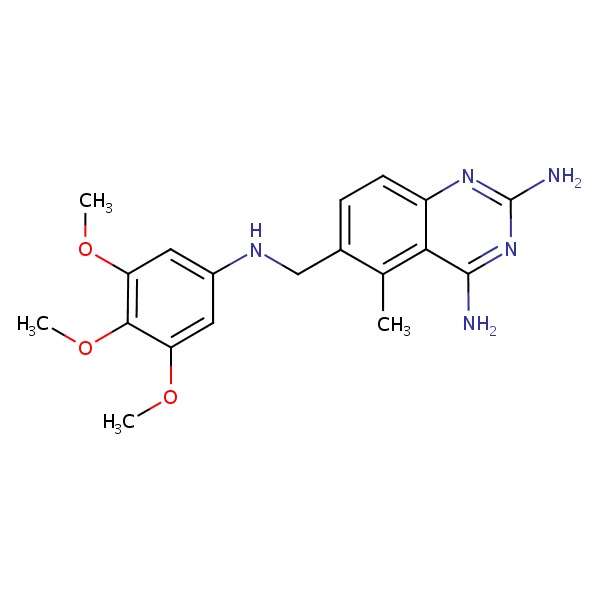 |
| Folate | 6484-89-5 | C19-H18-N7-O6.Na |
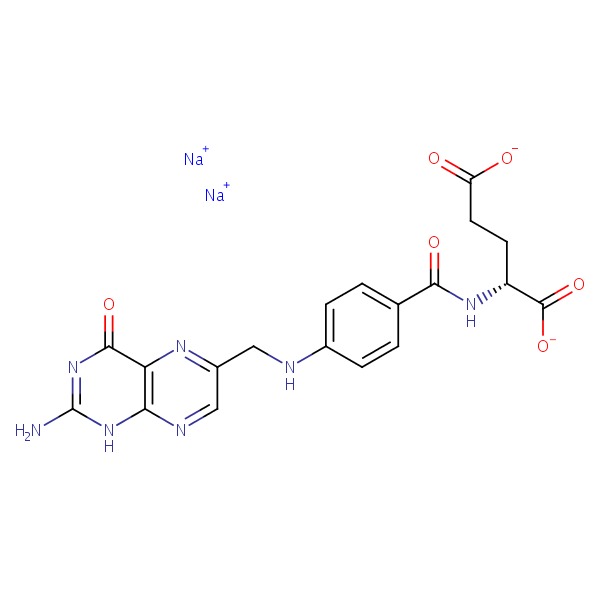 |
ANNOTATED BIBLIOGRAPHY
References updated: 18 April 2016
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; trimetrexate is listed as an antineoplastic agent that has not been associated with liver injury).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; trimetrexate is not discussed).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Folic acid analogs. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1690-94.(Textbook of pharmacology and therapeutics).
- Lin JT, Bertino JR. Trimetrexate: a second generation folate antagonist in clinical trial. J Clin Oncol 1987; 5: 2032-40. [PubMed: 2960788](Review of the chemical structure and mechanism of action of trimetrexate and summary of pilot studies in breast, lung and head and neck cancers, mentions that toxicity is largely myelosuppression, but gastrointestinal, renal and cutaneous adverse events can occur: “transient elevation of liver enzymes has also been reported”).
- Grem JL, Ellenberg SS, King SA, Shoemaker DD. Correlates of severe or life-threatening toxic effects from trimetrexate. J Natl Cancer Inst 1988; 80: 1313-8. [PubMed: 2971817](Analysis of side effects in 272 patients with cancer treated with trimetrexate suggested that leukopenia and mucositis were dose related and that patients with low baseline serum protein levels were more likely to suffer severe side effects; ALT or AST elevations above 5 times ULN occurring in 22% of subjects with serum albumin 3.2 g/dL or below, but only 5-9% with normal values).
- Balis FM, Patel R, Luks E, Doherty KM, Holcenberg JS, Tan C, Reaman GH, et al. Pediatric phase I trial and pharmacokinetic study of trimetrexate. Cancer Res 1987; 47: 4973-6. [PubMed: 2957048](Among 30 children with cancer treated with several doses of trimetrexate, dose limiting toxicities were myelosuppression, mucositis, rash and “mild, dose-related, transient elevations in serum transaminases [2- to 3-fold higher than baseline]”).
- Bertino JR. Trimetrexate: overall clinical results. Semin Oncol 1988; 15 (2 Suppl 2): 50-1. [PubMed: 2966986](Commentary on preliminary studies of trimetrexate in several solid tumors mentions promising results in lung, breast and head and neck cancers; toxicities described as very much like those with methotrexate).
- Allegra CJ, Kovacs JA, Drake JC, Swan JC, Chabner BA, Masur H. Activity of antifolates against Pneumocystis carinii dihydrofolate reductase and identification of a potent new agent. J Exp Med 1987; 165: 926-31. [PMC free article: PMC2188293] [PubMed: 2950200](In vitro studies of rat and P. jirovecii dihydrofolate reductase showed superior inhibitory activity of trimetrexate compared to trimethoprim and pyrimethamine and in vivo studies demonstrated its effectiveness in rat P. jirovecii infection).
- Allegra CJ, Chabner BA, Tuazon CU, Ogata-Arakaki D, Baird B, Drake JC, Simmons JT, et al. Trimetrexate for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med 1987; 317: 978-85. [PubMed: 2958710](Among 49 patients with HIV infection and Pneumocystis jirovecii pneumonia treated with trimetrexate and leucovorin, 63-71% responded including patients who were refractory or intolerant to conventional therapy; side effects were infrequent while ALT or AST elevations above 5 times ULN occurred in 4 [8%] patients, they resolved despite continuing therapy).
- Hughes WT. Pneumocystis carinii pneumonitis. N Engl J Med 1987; 317: 1021-3. [PubMed: 2958709](Editorial on Pneumocystis jirovecii infection in response to article by Allegra [1987: NEJM]).
- Eisenhauer EA, Zee BC, Pater JL, Walsh WR. Trimetrexate: predictors of severe or life-threatening toxic effects. J Natl Cancer Ins. 1988; 80: 1318-22. [PubMed: 2971818](Analysis of 68 patients treated with trimetrexate in cancer chemotherapy trials found that low serum protein levels and metastatic liver disease were predictive of likelihood of severe side effects).
- Trimetrexate for Pneumocystis carinii pneumonia. Med Lett Drugs Ther 1989; 31: 5-6. [PubMed: 2521370](Concise summary of the mechanism of action and efficacy of trimetrexate with leucovorin as therapy of Pneumocystis jirovecii pneumonia shortly after its approval for this use in the US, mentions that when combined with leucovorin, is well tolerated; no mention of ALT elevations).
- Sattler FR, Allegra CJ, Verdegem TD, Akil B, Tuazon CU, Hughlett C, Ogata-Arakaki D, et al. Trimetrexate-leucovorin dosage evaluation study for treatment of Pneumocystis carinii pneumonia. J Infect Dis 1990; 161: 91-6. [PubMed: 2136905](In a dose finding study of trimetrexate and leucovorin in 53 patients with Pneumocystis jirovecii pneumonia, ALT elevations above 5 times ULN occurred in 15 patients [28%] with peak values of 184 to 1205 U/L, but no patients developed jaundice, and all improved despite continuing therapy, none requiring early discontinuation because of liver injury).
- Grem JL, King SA, Costanza ME, Brown TD. Hypersensitivity reactions to trimetrexate. Invest New Drugs 1990; 8: 211-4. [PubMed: 2143501](5 patients had immediate hypersensitivity reactions to infusions of trimetrexate; symptoms included immediate hypotension, loss of consciousness, flushing, fever, and periorbital edema; all resolved with stopping and either corticosteroids or antihistamines or both; no mention of ALT elevations or hepatotoxicity).
- Helweg-Larsen J, Benfield T, Atzori C, Miller RF. Clinical efficacy of first- and second-line treatments for HIV-associated Pneumocystis jirovecii pneumonia: a tri-centre cohort study. J Antimicrob Chemother 2009; 64: 1282-90. [PMC free article: PMC2775667] [PubMed: 19858161](Among 1122 HIV infected patients with P. jirovecii pneumonia treated at 3 European centers between 1989 and 2004, most were treated with trimethoprim/sulfamethoxazole [81%] or pentamidine [7%] and few with trimetrexate/leucovorin; no discussion of adverse reactions).
- Short CE, Gilleece YC, Fisher MJ, Churchill DR. Trimetrexate and folinic acid: a valuable salvage option for Pneumocystis jirovecii pneumonia. AIDS 2009; 23: 1287-90. [PubMed: 19424049](Among 14 patients with refractory Pneumocystis jirovecii pneumonia treated with trimetrexate and leucovorin, the response rate was 71% and there were no early discontinuations for side effects; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Trimetrexate for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome.[N Engl J Med. 1987]Trimetrexate for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome.Allegra CJ, Chabner BA, Tuazon CU, Ogata-Arakaki D, Baird B, Drake JC, Simmons JT, Lack EE, Shelhamer JH, Balis F. N Engl J Med. 1987 Oct 15; 317(16):978-85.
- Review Trimetrexate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of Pneumocystis carinii pneumonia.[Drugs. 1995]Review Trimetrexate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of Pneumocystis carinii pneumonia.Fulton B, Wagstaff AJ, McTavish D. Drugs. 1995 Apr; 49(4):563-76.
- Trimetrexate and folinic acid: a valuable salvage option for Pneumocystis jirovecii pneumonia.[AIDS. 2009]Trimetrexate and folinic acid: a valuable salvage option for Pneumocystis jirovecii pneumonia.Short CE, Gilleece YC, Fisher MJ, Churchill DR. AIDS. 2009 Jun 19; 23(10):1287-90.
- Efficacy of trimetrexate, a potent lipid-soluble antifolate, in the treatment of rodent Pneumocystis carinii pneumonia.[Am J Trop Med Hyg. 1988]Efficacy of trimetrexate, a potent lipid-soluble antifolate, in the treatment of rodent Pneumocystis carinii pneumonia.Kovacs JA, Allegra CJ, Kennedy S, Swan JC, Drake J, Parrillo JE, Chabner B, Masur H. Am J Trop Med Hyg. 1988 Nov; 39(5):491-6.
- Review Pralatrexate.[LiverTox: Clinical and Researc...]Review Pralatrexate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Trimetrexate - LiverToxTrimetrexate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...