NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tobramycin is a parenterally administered, broad spectrum aminoglycoside antibiotic that is widely used in the treatment of moderate to severe bacterial infections due to sensitive organisms. Despite its wide use, tobramycin has rarely been linked to instances of clinically apparent liver injury.
Background
Tobramycin (toe" bra mye' sin) is a semisynthetic aminoglycoside, similar in spectrum of activity to gentamicin, that is widely used for severe bacterial infections caused by sensitive agents, primarily aerobic gram negative bacteria. Like other aminoglycosides, tobramycin is thought to act by binding to bacterial ribosomes and inhibiting protein synthesis. Nevertheless, tobramycin is considered bacteriocidal as well as bacteriostatic. Tobramycin and other aminoglycosides are typically used in combination with a penicillin or cephalosporin for treatment of severe infections with E. coli, Staphylococcus aureus, Enterobacter, Klebsiella, Serratia, Pseudomonas aeruginosa, and other gram negative bacteria resistant to less toxic antibiotics. Tobramycin is most commonly used for septicemia, bacterial endocarditis, peritonitis, meningitis, pelvic inflammatory disease and pneumonia. Tobramycin was approved for use in the United States in 1980 and is available in multiple generic forms in parenteral formulations; typical adult doses are 3 mg/kg per day im or iv, usually in three divided doses, often after an initial loading dose. The dose of tobramycin must be modified based upon renal function and monitoring of drug levels is advisable. Pediatric formulations, nebulizer solutions for inhalation, and topical formulations for ophthalmologic use are also available. Common side effects include dizziness, headache, confusion, nausea and skin rash. Important, dose related adverse effects include oto- and nephrotoxicity, which are shared by all aminoglycosides.
Hepatotoxicity
Intravenous and intramuscular therapy with tobramycin is usually associated with no increase in rates of serum aminotransferase or bilirubin elevations. Only isolated case reports of acute liver injury with jaundice have been associated with aminoglycoside therapy including tobramycin, not all of which are very convincing. The hepatic injury is typically mixed but can evolve into a cholestatic hepatitis. The latency to onset is rapid, occurring within 1 to 3 weeks and is typically associated with skin rash, fever and sometimes eosinophilia. Recovery typically occurs within 1 to 2 months and chronic injury has not been described. Aminoglycosides are not mentioned in large case series of drug induced liver disease and acute liver failure; thus, hepatic injury due to tobramycin is rare if it occurs at all.
Mechanism of Injury
The cause of symptomatic, icteric hepatic injury from tobramycin is not known, but is likely to be immunoallergic. Uptake of the aminoglycosides into hepatocytes is limited and they are rapidly excreted in the urine; high concentrations are found mainly in renal tubular cells and hair cells of the inner ear, perhaps explaining why they are more likely to cause nephro- or oto- rather than hepatotoxicity.
Outcome and Management
The outcome of hepatic injury due to aminoglycosides is usually benign. Acute liver failure has not been associated with tobramycin use and no convincing cases of chronic bile duct vanishing syndrome have been described after its use. Patients with tobramycin hepatotoxicity should probably avoid all systemic aminoglycoside use.
References to the safety and potential hepatotoxicity of tobramycin are provided in the Overview section on the Aminoglycosides.
Drug Class: Aminoglycosides
Other Drugs in the Class: Amikacin, Gentamicin, Neomycin, Plazomicin, Streptomycin
CASE REPORT
Case 1. Acute hepatitis arising during tobramycin therapy.
[Modified from: Nisly SA, Ray SM, Moye RA. Tobramycin-induced hepatotoxicity. Ann Pharmacother 2007; 41: 2061-5. PubMed Citation]
A 20 year old woman with osteomyelitis was treated with intravenous tobramycin and ceftazidime and developed rising levels of ALT and AST with mild jaundice. The patient had a past history of surgery on her right femur and recurrent osteomyelitis. She presented with fever and chills and was started on intravenous piperacillin/tazobactam, ciprofloxacin and vancomycin, which was changed to intravenous ceftazidime and tobramycin when blood cultures revealed P. aeruginosa, sensitive to this regimen. Serum ALT, AST and Alk P levels started to rise (see Table), and piperacillin/tazobactam was substituted for ceftazidime. Tests for hepatitis A, B and C were negative. Imaging showed no evidence of gallstones or biliary obstruction. Because of further increases in liver tests, aztreonam was substituted for piperacillin/tazobactam. When serum bilirubin began to rise, all antibiotics were stopped. Thereafter, liver tests began to improve, and they continued downward even with reinitiation of intravenous aztreonam and ciprofloxacin. Serum aminotransferase levels were near normal by the time of discharge.
Key Points
| Medication: | Tobramycin, 70-100 mg every 8 hours for 9 days |
| Pattern: | Mixed (R=~3) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 3-4 days to ALT elevations, 8 days to jaundice |
| Recovery: | One week |
| Other medications: | Paroxetine, alprazolam, hydrocodone/acetaminophen, oxycodone, hydromorphone, promethazine, docusate, metoclopramide, piperacillin/tazobactam, ciprofloxacin, aztreonam, and ceftazidime |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 48 | 220 | 3.2 | Admission with fever | |
| 1 day | 40 | 200 | Started tobramycin/ceftazidime | ||
| 6 days | 139 | 432 | 1.8 | Piperacillin/tazobactam started | |
| 8 days | 315 | 330 | 4.9 | Aztreonam started | |
| 10 days | 330 | 320 | All antibiotics stopped | ||
| 12 days | 0 | 210 | 225 | ||
| 13 days | 1 days | 180 | 210 | ||
| 14 days | 2 days | 160 | 200 | Aztreonam/ciprofloxacin started | |
| 15 days | 3 days | 110 | 180 | ||
| 17 days | 5 days | 65 | 158 | 1.5 | |
| Normal Values | <55 | <148 | <1.2 | ||
Comment
While the timing of onset and subsequent improvement in serum aminotransferase and alkaline phosphatase levels suggest that tobramycin might be the cause of the liver injury, the history is complex. The patient was mildly jaundiced and had evidence for underlying liver disease at the time of admission before antibiotic therapy (this may have been due to sepsis). Furthermore, she received a multitude of medications, including several other antibiotics that are capable of causing liver injury (piperacillin, ciprofloxacin, ceftazidime). Because of the polypharmacy and presence of sepsis, the likelihood that the injury was due to tobramycin might be rated as only possible.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tobramycin – Generic
DRUG CLASS
Aminoglycosides
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
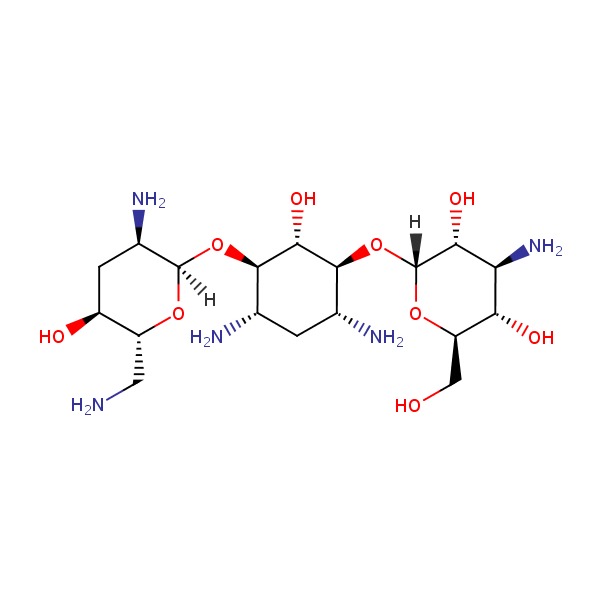
| Tobramycin | 32986-56-4 | C18-H37-N5-O9 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Gentamicin.[LiverTox: Clinical and Researc...]Review Gentamicin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Plazomicin.[LiverTox: Clinical and Researc...]Review Plazomicin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Amikacin.[LiverTox: Clinical and Researc...]Review Amikacin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Concentrated biosynthesis of tobramycin by genetically engineered Streptomyces tenebrarius.[J Gen Appl Microbiol. 2014]Concentrated biosynthesis of tobramycin by genetically engineered Streptomyces tenebrarius.Xiao J, Li H, Wen S, Hong W. J Gen Appl Microbiol. 2014; 60(6):256-61.
- Surveillance of aminoglycoside resistance. European data.[Am J Med. 1986]Surveillance of aminoglycoside resistance. European data.Van Landuyt HW, Boelaert J, Glibert B, Gordts B, Verbruggen AM. Am J Med. 1986 Jun 30; 80(6B):76-81.
- Tobramycin - LiverToxTobramycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...