NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The thrombopoietin receptor agonists mimic the action of thrombopoietin on its receptor and stimulate the activation, proliferation and maturation of megakaryocytes, resulting in an increase in circulating platelet counts. Thrombopoietin itself acts in this manner, but when recombinant thrombopoietins were used clinically, they were found to cause rebound thrombocytopenia, probably due to induction of anti-thrombopoietin antibodies. For this reason, direct administration of thrombopoietin was abandoned as an approach to treating thrombocytopenia and other approaches to activating the thrombopoietin receptor were sought.
Several thrombopoietin receptor agonists were subsequently developed and are now in clinical use for chronic idiopathic thrombocytopenic purpura (ITP) and for raising platelet counts in persons with thrombocytopenia undergoing surgical procedures or other thrombocytopenic conditions. Eltrombopag, lusutrombopag and avatrombopag are peptide-like, small molecular weight agonists of the thrombopoietin receptor. These agents are given by mouth and result in significant increases in platelet counts in normal persons as well as patients with thrombocytopenia due to hematologic and liver diseases. Romiplostim, in contrast, is a recombinant polypeptide that binds to and activates the thrombopoietin receptor despite having no amino acid homology to native thrombopoietin. It also increases platelet counts in normal subjects as well as patients with chronic ITP but has not been associated with induction of anti-thrombopoietin antibodies.
ELTROMBOPAG
Background
Eltrombopag (el trom' boe pag) is a small molecular weight peptide-like molecule that binds to the transmembrane domain of the thrombopoietin receptor and causes its activation and the proliferation and differentiation of megakaryocytes, with a resultant increase in synthesis and release of platelets. In multiple clinical trials, eltrombopag was shown to raise the platelet count in patients with idiopathic thrombocytopenic purpura (ITP), aplastic anemia and cirrhosis due to chronic hepatitis C during interferon therapy. Eltrombopag was the first oral thrombopoietin receptor agonist approved for use in the United States, initially for treatment of ITP iin 2008. The indications have subsequently been expanded to other thrombocytopenic conditions. Eltrombopag is available as tablets of 12.5, 25, 50, 75 and 100 mg under the brand name Promacta. The typical dose is 25 to 50 mg once daily by mouth. The most common side effects include nausea, diarrhea, fatigue, muscle aches, headaches and dizziness. Rare, but potentially serious adverse reactions include vascular occlusions, stroke and myocardial infarction.
Hepatotoxicity
In clinical trials in patients with ITP, ALT elevations occurred in 10% to 11% of eltrombopag vs 3% to 7% of placebo treated subjects, but the elevations were usually mild and transient, resolving once eltrombopag was discontinued and sometimes even with continued use. Instances in which there was recurrence of serum enzyme elevations with restarting eltrombopag have been reported and several patients were said to have developed serious liver disease on treatment, perhaps as a result of portal vein thrombosis. Because of such reports, eltrombopag has a boxed warning about hepatotoxicity and the possibility of hepatic decompensation when treating patients with chronic hepatitis C, in which situation monitoring of liver tests is recommended. Despite this, there have been no published reports of idiosyncratic clinically apparent liver injury attributable to eltrombopag therapy in the medical literature and the clinical characteristics, timing on onset, pattern of enzyme elevations and response to withdrawal of therapy of the liver injury attributed to the drug have not been described. Furthermore, with the development of more potent antiviral agents for hepatitis C, interferon is now rarely used and the indication for concurrent use of eltrombopag for thrombocytopenia during interferon therapy is rarely encountered.
Likelihood score: E* (suspected, but unproven cause of clinically apparent liver injury).
Mechanism of Injury
A mechanism of injury that might lead to serum enzyme elevations during eltrombopag therapy is not known. The thrombosis induced by thrombopoietin receptor agonists might cause a hypercoagulable state resulting in portal vein or hepatic vein thrombosis. However, patients with cirrhosis are known to be at increased risk for portal vein thrombosis, independent of thrombopoiesis-stimulating agents. Eltrombopag is metabolized in the liver, largely by the cytochrome P450 system (CYP 1A2, 2C8) and by the uridine diphosphate glucuronosyltransferase transport system (UGT1A1 and 1A3) and can have significant drug-drug interactions, particularly with statins. Eltrombopag should also be taken on an empty stomach at least 1 hour before and 2 hours after a meal.
Outcome and Management
Serum aminotransferase elevations above 3 times the upper limit of normal (if confirmed and persistent) during eltrombopag therapy should lead to dose reduction or temporary cessation. Enzyme elevations accompanied by jaundice or symptoms should lead to immediate discontinuation. Eltrombopag has not been implicated in cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome, but has been linked to clinical worsening or decompensation in patients with cirrhosis due to hepatitis C. For this reason, testing for serum aminotransferase and bilirubin levels is recommended before starting eltrombopag and monitoring for changes if symptoms or signs of liver injury arise. There is no reason to suspect any degree of cross sensitivity in risk for hepatic injury among the various thrombopoietin receptor agonists or other hematologic growth factors.
AVATROMBOPAG
Background
Avatrombopag (ah" va trom' boe pag) was the second oral thrombopoietin receptor agonist approved as therapy of thrombocytopenia in the United States. Like eltrombopag, avatrombopag is a small molecular weight peptide-like molecule that binds to the thrombopoietin receptor and causes its activation and the proliferation and differentiation of megakaryocytes, with a resultant increase in synthesis and release of platelets. In clinical trials, avatrombopag was shown to raise the platelet count in patients with thrombocytopenia due to cirrhosis and to reduce platelet transfusion requirements during elective invasive procedures. Avatrombopag was approved for use in the United States in 2018 as therapy of thrombocytopenia in adults with chronic liver disease undergoing surgical, radiologic or medical invasive procedures. Avatrombopag is available as tablets of 20 mg under the brand name Doptelet. The typical dose regimen is 40 or 60 mg once daily for 5 days in preparation of a surgical or invasive procedure 5 to 8 days after the last dose. The most common side effects include fever, abdominal pain, nausea, headache, fatigue and peripheral edema. Rare, but potentially serious adverse reactions include arterial or venous thromboses.
Hepatotoxicity
In clinical trials in patients with liver disease treated with 5 days of avatrombopag or placebo before undergoing procedures, ALT elevations occurred in 1% to 4% of avatrombopag vs 0% to 2% of control subjects, but the elevations were usually mild and transient, resolving once avatrombopag was discontinued. In prelicensure clinical trials, portal vein thrombosis occurred in some patients after avatrombopag therapy, but the frequency was low and only minimally higher than with placebo treatment. Since its approval, there have been no published reports of clinically apparent liver injury attributable to avatrombopag therapy but it has had limited clinical use. In clinical trials of long term use of avatrombopag, such as in ITP, serum enzyme elevations are more frequent.
Likelihood score: E* (suspected, but unproven cause of clinically apparent liver injury).
Mechanism of Injury
A mechanism of injury that might lead to serum enzyme elevations during avatrombopag therapy is not known. The thrombosis induced by thrombopoietin receptor agonists might cause a hypercoagulable state resulting in portal vein or hepatic vein thrombosis. However, patients with cirrhosis are known to be at increased risk for portal vein thrombosis, independent of thrombopoiesis-stimulating agents. Avatrombopag is metabolized in the liver, largely by the cytochrome P450 system (CYP 3A4, 2C9) and is a weak inducer of 2CP 2C8 and 2C9, but it has not been found to have clinically significant drug-drug interactions with microsomal enzyme modulators.
Outcome and Management
Serum aminotransferase elevations above 3 times the upper limit of normal (if confirmed and persistent) during avatrombopag therapy should lead to dose reduction or temporary cessation. Enzyme elevations accompanied by jaundice or symptoms should lead to immediate discontinuation. However, because avatrombopag is given for 5 days only, such elevations are quite rare. Avatrombopag has not been implicated in cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome.
LUSUTROMBOPAG
Background
Lusutrombopag (loo" soo trom' boe pag) was the third oral thrombopoietin receptor agonist approved as therapy of thrombocytopenia in the United States. Like eltrombopag and avatrombopag, lusutrombopag is a small molecular weight peptide-like molecule that binds to the thrombopoietin receptor and causes its activation and the proliferation and differentiation of megakaryocytes, with a resultant increase in synthesis and release of platelets. In several clinical trials, lusutrombopag was shown to raise the platelet count in patients with chronic liver disease and cirrhosis and was associated with lower platelet transfusion requirements in cirrhotic patients undergoing invasive surgical, radiologic and medical procedures. Lusutrombopag was approved for use in the United States in 2018 for treatment of thrombocytopenia in adults with chronic liver disease who are scheduled to undergo an invasive procedure. Lusutrombopag is available as tablets of 3 mg under the brand name Mulpleta. The typical dose is 3 mg once daily for 7 days in preparation for a scheduled invasive procedure 2 to 8 days after the last dose. Common side effects may include fever, abdominal pain, nausea, headache, fatigue and peripheral edema. Rare, but potentially serious adverse reactions include arterial or venous occlusions.
Hepatotoxicity
In clinical trials in patients with liver disease treated with 7 days of lusutrombopag or placebo before undergoing procedures, ALT elevations occurred in 1% of lusutrombopag vs no control subjects, but the elevations were mild and transient, resolving once lusutrombopag was discontinued. In prelicensure clinical trials, portal vein thrombosis occurred in some patients after lusutrombopag therapy, but the frequency was low and similar to that with placebo treatment. Since its approval, there have been no published reports of clinically apparent liver injury attributable lusutrombopag therapy but it has had limited clinical use.
Likelihood score: E* (suspected, but unproven cause of clinically apparent liver injury).
Mechanism of Injury
A mechanism of injury that might lead to serum enzyme elevations during lusutrombopag therapy is not known. The thrombosis induced by thrombopoietin receptor agonists might cause a hypercoagulable state resulting in portal vein or hepatic vein thrombosis. However, patients with cirrhosis are known to be at increased risk for portal vein thrombosis, independent of thrombopoiesis-stimulating agents. Lusutrombopag is metabolized in the liver to a small extend, largely by oxidation and glucuronidation. It does not induce cytochrome P450 enzymes and has not been implicated in clinically significant drug-drug interactions.
Outcome and Management
Serum aminotransferase elevations above 3 times the upper limit of normal (if confirmed and persistent) during lusutrombopag therapy should lead to dose reduction or temporary cessation. Enzyme elevations accompanied by jaundice or symptoms should lead to immediate discontinuation. Because it is typically given for 7 days only, such abnormalities are uncommon and rarely require intervention. Lusutrombopag has not been implicated in cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome.
ROMIPLOSTIM
Background
Romiplostim (roe" mi ploe' stim) is a recombinant fusion protein consisting of four linear 14 amino acid peptides fused to the Fc fragment of the human IgG1 heavy chain that binds to the thrombopoietin receptor, despite lack of homology with native thrombopoietin. The fusion polypeptide has a prolonged half-life, but must be administered parenterally. In several clinical trials, romiplostim was shown to raise platelet counts in patients with chronic ITP without inducing anti-thrombopoietin antibodies. Romiplostim was approved for use in ITP in the United States in 2008 and indications have subsequently been expanded to other thrombocytopenic conditions. Romiplostim is available as a solution in vials of 250 and 500 mcg/mL. The typical initial dose is 1 mcg/kg weekly by subcutaneous injection, which can be increased to a maximum of 10 mcg/kg based upon tolerance and effect. The most common side effects include nausea, diarrhea, fatigue, muscle aches, headaches and dizziness. Rare, but potentially serious adverse reactions include vascular occlusions, stroke and myocardial infarction.
Hepatotoxicity
In clinical trials in patients with ITP, romiplostim was not linked to ALT elevations or to episodes of clinically apparent liver injury. Since its approval and more widespread use, romiplostim has not been linked to cases of hepatic injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
A mechanism of injury by which romiplostim might cause liver injury is not known. It is a recombinant polypeptide and is likely metabolized by many tissues including the cells on which it acts. Romiplostim has not been linked to serious drug-drug interactions.
Outcome and Management
Romiplostim has not been implicated in cases of clinically apparent liver injury, liver failure or chronic hepatitis. Patients who have developed serum enzyme elevations during treatment with eltrombopag have been successfully and safely switched to romiplostim therapy.
Drug Class: Hematologic Growth Factors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Avatrombopag – Doptelet®
Eltrombopag – Promacta®
Lusutrombopag – Mulpleta®
Romiplostim – Nplate®
DRUG CLASS
Hematologic Growth Factors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Avatrombopag | 570406-98-3 | C29-H34-Cl2-N6-O3-S2 |
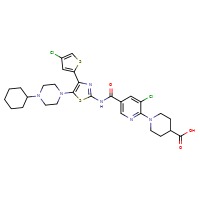 |
| Eltrombopag | 496775-61-2 | C25-H22-N4-O4 |
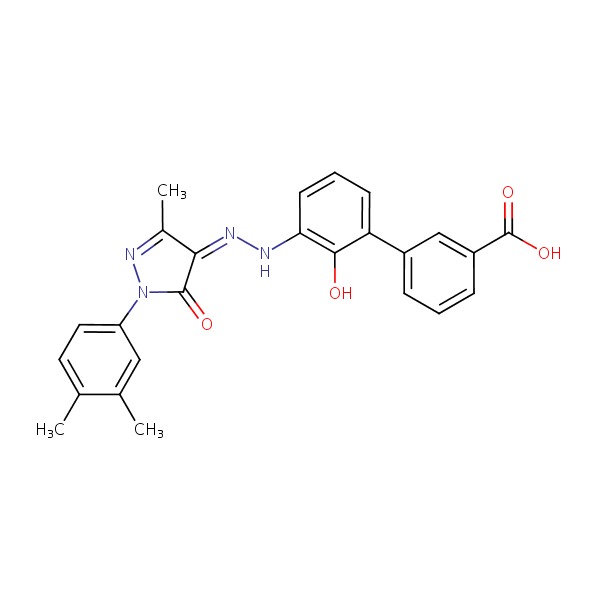 |
| Lusutrombopag | 1110766-97-6 | C29-H32-Cl2-N2-O5-S |
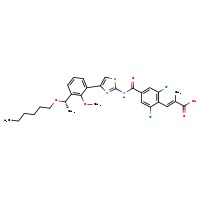 |
| Romiplostim | 267639-76-9 | Protein | Not Available |
| Thrombopoietin | 9014-42-0 | Protein | Not Available |
ANNOTATED BIBLIOGRAPHY
References updated: 30 December 2018
Abbreviations: ITP, idiopathic thrombocytopenic purpura.
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp 555-88.(Review of hepatotoxicity published in 1999; the thrombopoietin receptor agonists are not specifically mentioned).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; thrombopoietin receptor agonists are not discussed).
- Kaushansky K, Kipps TJ. Hematopoietic agents: growth factors, minerals and vitamins. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 751-67.(Textbook of pharmacology and therapeutics).
- Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 2001; 98: 3241-8. [PubMed: 11719360](Analysis of 3 subjects who developed thrombocytopenia [<10,000] after 2 to 28 injections of a commercial pegylated recombinant thrombopoietin; identified antibodies to thrombopoietin as the probable cause).
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: double-blind randomised controlled trial. Lancet 2008; 371: 395-403. [PubMed: 18242413](Among 125 patients with ITP treated with standard of case or romiplostim for 24 weeks, side effects more common with the drug included dizziness, insomnia, abdominal and muscle pain; no mention of ALT elevations or hepatotoxicity).
- Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009; 113 (10): 2161-71. [PubMed: 18981291](Among 142 patients with chronic ITP who were treated with romiplostim for up to 3 years, platelet count responses [>50,000] occurred in 87%, but there were 5 serious thrombotic events including one case of portal vein thrombosis).
- Two new drugs for chronic ITP. Med Lett Drugs Ther 2009; 51 (1305): 10-1. [PubMed: 19197233](Concise review of the mechanism of action, clinical efficacy, safety and costs of romiplostim and eltrombopag after their approval for use in ITP in the US mentions that liver function test abnormalities occurred in 10% of eltrombopag treated vs 8% of placebo treated patients, and that one developed “grade 4 liver function test abnormalities and died”).
- Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med 2010; 363: 1889-99. [PubMed: 21067381](Among 234 adults with ITP treated with standard of care or romiplostim, bleeding episodes as well as need for splenectomy and blood transfusions were lower in romiplostim treated subjects; one patient receiving standard of care died of hepatic failure; no mention of ALT elevations or hepatotoxicity).
- Kuter DJ. Biology and chemistry of thrombopoietic agents. Semin Hematol 2010 Jul; 47 (3): 243-8. [PMC free article: PMC2904343] [PubMed: 20620435](Review of the structure and mechanism of action of thrombopoietin and the development of two mimetic drugs [eltrombopag and romiplostim] for use in chronic ITP).
- Khellaf M, Michel M, Quittet P, Viallard JF, Alexis M, Roudot-Thoraval F, Cheze S, et al. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood 2011; 118: 4338-45. [PubMed: 21832276](Among 72 patients with ITP treated with romiplostim for at least 2 years, long term platelet response occurred in 65% of patients and common side effects were arthralgias, fatigue and nausea; no mention of ALT elevations or hepatotoxicity).
- Cooper N, Terrinoni I, Newland A. The efficacy and safety of romiplostim in adult patients with chronic immune thrombocytopenia. Ther Adv Hematol 2012; 3: 291-8. [PMC free article: PMC3627322] [PubMed: 23616916](Review of the efficacy and safety of romiplostim in treatment of adults with ITP; no mention of ALT elevations or hepatotoxicity).
- Cheng G. Eltrombopag, a thrombopoietin- receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol 2012; 3: 155-64. [PMC free article: PMC3573439] [PubMed: 23556122](Review of the efficacy and safety of eltrombopag based upon 5 prospective trials in ITP, reported serum ALT elevations in 10% of eltrombopag vs 3% of placebo recipients, but elevations were usually asymptomatic and transient, often resolving spontaneously; no episodes of serious clinically apparent liver injury).
- Tsukamoto S, Nakaseko C, Takeuchi M, Kumagai K, Komatsu T, Tanaka H, Hara S, et al. Safety and efficacy of romiplostim in patients with eltrombopag-resistant or -intolerant immune thrombocytopenia. Br J Haematol 2013; 163: 286-9. [PubMed: 23862773](Among 3 patients with ITP who developed ALT elevations during eltrombopag therapy and who were switched to romiplostim, the liver dysfunction resolved in all).
- Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, Viallard JF, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol 2013; 161: 411-23. [PubMed: 23432528](Among 292 patients with ITP treated with romiplostim for up to 5 years, thrombotic events occurred in 6.5% including one case of portal vein thrombosis, whose platelet count was 473,000 three days before the event).
- Rodeghiero F, Stasi R, Giagounidis A, Viallard JF, Godeau B, Pabinger I, Cines D, et al. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: a pooled analysis of 13 clinical trials. Eur J Haematol 2013; 91 (5): 423-36. [PubMed: 23927437](A pooled analysis of long term studies of romiplostim in 653 patients with ITP reported that thrombotic events occurred in 6% on romiplostim and 3.6% on placebo; no mention of ALT elevations or clinically apparent liver injury).
- Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol 2014; 165: 248-58. [PubMed: 24528208](History of the development of agents to increase platelet counts focusing upon eltrombopag and romiplostim).
- Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol 2014; 165: 259-68. [PubMed: 24499199](History of discovery of thrombopoietin and evolution of knowledge about its mechanism of action and development of drugs that activate its receptor).
- Prica A, Sholzberg M, Buckstein R. Safety and efficacy of thrombopoietin-receptor agonists in myelodysplastic syndromes: a systematic review and meta-analysis of randomized controlled trials. Br J Haematol 2014; 167: 626-38. [PubMed: 25155450](Systematic review identified 5 trials of thrombopoietin receptor agonists in 384 patients with myelodysplastic syndromes; no discussion or mention of ALT elevations or hepatotoxicity in review of safety).
- Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ, Kim HJ. Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia. Blood Res 2015; 50: 19-25. [PMC free article: PMC4377333] [PubMed: 25830126](Among 18 Korean patients with refractory ITP treated with eltrombopag, ALT elevations occurred in 5 and were greater than 5 times ULN in 2, but none required drug discontinuation or developed clinically apparent liver injury).
- Platzbecker U, Wong RS, Verma A, Abboud C, Araujo S, Chiou TJ, Feigert J, et al. Safety and tolerability of eltrombopag versus placebo for treatment of thrombocytopenia in patients with advanced myelodysplastic syndromes or acute myeloid leukaemia: a multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial. Lancet Haematol 2015: e417-26. [PubMed: 26686043](Among 98 patients with low platelet counts due to myelodysplastic syndromes treated with eltrombopag vs placebo, ALT elevations occurred in 9% of treated subjects, but only 1 was above 5 times ULN).
- Grainger JD, Locatelli F, Chotsampancharoen T, Donyush E, Pongtanakul B, Komvilaisak P, Sosothikul D, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet 2015; 386 (10004): 1649-58. [PubMed: 26231455](Among 92 children with chronic ITP treated with eltrombopag or placebo for 13 weeks, adverse events included AST elevations in 6% vs 0%, but only 1 patient had elevations above 5 times ULN).
- Cines DB, Gernsheimer T, Wasser J, Godeau B, Provan D, Lyons R, Altomare I, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol 2015; 102: 259-70. [PubMed: 26201709](Pooled analysis of 1059 patients in 14 trials of romiplostim mentions that common side effects were headache and epistaxis and serious adverse events include thromboses and bleeding including 4 cases of portal vein thrombosis; but there were no liver related serious adverse events or deaths).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to eltrombopag or romiplostim).
- Vishnu P, Aboulafia DM. Long-term safety and efficacy of romiplostim for treatment of immune thrombocytopenia. J Blood Med 2016; 7: 99-106. [PMC free article: PMC4888762] [PubMed: 27307776](Review of long term safety of romiplostim discusses common symptoms of headache, fatigue, epistaxis and arthralgia, but does not mention ALT elevations or hepatotoxicity).
- Zaja F, Barcellini W, Cantoni S, Carpenedo M, Caparrotti G, Carrai V, Di Renzo N, et al. Thrombopoietin receptor agonists for preparing adult patients with immune thrombocytopenia to splenectomy: results of a retrospective, observational GIMEMA study. Am J Hematol 2016; 91: E293-5. [PubMed: 26910388](Among 31 patients with ITP treated with eltrombopag [n=7] or romiplostim [n=24] in preparation of surgery, platelet counts rose from a mean of 11,000 to 114,000/uL, and 29 underwent splenectomy, one developing portal vein thrombosis and one pulmonary embolus).
- Kurokawa T, Murata S, Ohkohchi N. Stable liver function during long-term administration of eltrombopag, a thrombopoietin receptor agonist, in patients with chronic liver disease. Tohoku J Exp Med 2016; 240: 277-9. [PubMed: 27928110](Among 5 patients with chronic liver disease and thrombocytopenia treated with eltrombopag for 6 months, all had improvements in platelet counts without worsening of ALT or bilirubin levels).
- González-López TJ, Fernández-Fuertes F, Hernández-Rivas JA, Sánchez-González B, Martínez-Robles V, Alvarez-Román MT, Pérez-Rus G, et al. Efficacy and safety of eltrombopag in persistent and newly diagnosed ITP in clinical practice. Int J Hematol 2017; 106: 508-16. [PubMed: 28667351](Among 220 Spanish adults with ITP treated with eltrombopag for up to 15 months, clinical responses occurred in 80% and 10 [5%] had serum enzyme elevations, all of which resolved on dose modification or discontinuation and none were accompanied by symptoms or jaundice).
- Hwang YY, Gill H, Chan TSY, Leung GMK, Cheung CYM, Kwong YL. Eltrombopag in the management of aplastic anaemia: real-world experience in a non-trial setting. Hematology 2018 Jan 5:1-6. [PubMed: 29303047](Among 20 patients with aplastic anemia treated with eltrombopag, 90% had an objective response, but all also had skin discoloration; no mention of ALT elevations or hepatotoxicity).
- Terrault NA, Hassanein T, Howell CD, Joshi S, Lake J, Sher L, Vargas H, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol 2014; 61: 1253-9. [PubMed: 25048952](Among 130 patients with cirrhosis and thrombocytopenia undergoing an elective procedure who were treated with 3 different dose-regimens of avatrombopag or placebo for 7 days, platelet counts rose in a greater proportion of avatrombopag than placebo recipients and 1 subject developed portal vein thrombosis 4 weeks after avatrombopag treatment).
- Bussel JB, Kuter DJ, Aledort LM, Kessler CM, Cuker A, Pendergrass KB, Tang S, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood 2014; 123: 3887-94. [PubMed: 24802775](Among 64 patients with chronic ITP treated with different doses of avatrombopag or placebo for 28 days, platelet counts rose in 13-80% of active drug- vs 0% of placebo-recipients and elevations were maintained for 24 weeks in an extension phase, while adverse were mostly mild-to-moderate and there were no reported severe hepatic adverse events).
- Bussel JB, de Miguel PG, Despotovic JM, Grainger JD, Sevilla J, Blanchette VS, Krishnamurti L, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol 2015; 2: e315-25. [PubMed: 26688484](Among 67 children with chronic ITP enrolled in a 3-phase controlled study, eltrombopag recipients were more likely to have a platelet response and have reduced bleeding episodes than those on placebo, and adverse event rates were similar, but 3 subjects on eltrombopag had ALT elevations above 3 times ULN but all resolved, 2 upon discontinuation and one without dose modification).
- Eltrombopag (Revolade ) and thrombocytopenia in patients with hepatitis C. hepatotoxic drug; more harms than benefits. Prescrire Int 2015; 24: 208-9. [PubMed: 26417629](Commentary on reports to national registries of hepatic decompensation in patients with chronic hepatitis C receiving eltrombopag while being treated with peginterferon and ribavirin, suggesting caution in its use).
- Choy KW, Wijeratne N, Doery JC. Eltrombopag: liver toxicity, kidney injury or assay interference? Pathology 2016; 48: 754-6. [PubMed: 27780601](Eltrombopag is a highly colored drug which is brown in alkaline and yellow in acidic pH, colors which can interfere with spectrophotometric assays for bilirubin, creatinine and phosphate).
- Kim ES. Lusutrombopag: First global approval. Drugs 2016; 76: 155-8. [PubMed: 26666417](Review of the mechanism of action, history of development, pharmacology, clinical efficacy and safety of lusutrombopag; no mention of ALT elevations or hepatotoxicity).
- Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, Allen LF, Hassanein T. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 2018; 155: 705-18. [PubMed: 29778606](Among 435 pateints with chronic liver disease and thrombocytopenia treated with 5 days of oral avatrombopag or placebo before undergoing an elective procedure, platelet counts rose higher and need for platelet transfusions were less with active drug than placebo, while adverse event rates were similar although 2 patients receiving avatrombopag developed portal vein thrombosis and 2 died [hepatic coma and multiorgan failure]).
- Jurczak W, Chojnowski K, Mayer J, Krawczyk K, Jamieson BD, Tian W, Allen LF. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol 2018; 183: 479-90. [PMC free article: PMC6282556] [PubMed: 30191972](Among 49 adults with ITP in a 26-week randomized controlled trial, platelet counts increased in those on avatrombopag but not on placebo, and “assessment of …clinical laboratory evaluations… identified no safety signal”; one patient had ALT elevations above 5 times ULN but had underlying fatty liver and hepatitis C).
- Shirley M. Avatrombopag: first global approval. Drugs 2018 Jul 11. [Epub ahead of print] [PubMed: 29995177](Review of the history of development, mechanism of action, clinical efficacy and safety of avatrombopag shortly after its approval in the US; mentions that 2 cases of portal vein thrombosis occurred in the trials of its use in patients with chronic liver disease, but "there was no evidence... that avatrombopag was associated with hepatotoxicity").
- Marano M, Serafinelli J, Cairoli S, Martinelli D, Pisani M, Palumbo G, Cefalo MG, et al. Eltrombopag-induced acute liver failure in a pediatric patient: a pharmacokinetic and pharmacogenetic analysis. Ther Drug Monit 2018; 40: 386-8. [PubMed: 29683873](3 year old girl with ITP treated with eltrombopag for 6 months developed lactic acidosis, hyperammonemia and abnormal liver tests [bilirubin 2.2 mg/dL, ALT 293 U/L, INR 9.2] with high serum eltrombopag levels, recovering after stopping drug and later found to have variant alleles in drug metabolizing enzymes, CYP2C8, UGT1A1 and ABCG2).
- Loffredo L, Violi F. Thrombopoietin receptor agonists and risk of portal vein thrombosis in patients with liver disease and thrombocytopenia: A meta-analysis. Dig Liver Dis 2018 Jun 20. pii: S1590-8658(18)30792-8. [PubMed: 29958825](Among 1953 patients with chronic liver disease and thrombocytopenia treated with a thrombopoietin receptor agonist in 4 randomized controlled trials, the pooled incidence of portal vein thrombosis was 1.6% on active drug vs 0.6% on placebo [p=0.055], while rates of arterial and venous thromboembolic events were 3.6% vs 1.1% [p=0.003]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Hematologic Growth Factors.[LiverTox: Clinical and Researc...]Review Hematologic Growth Factors.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Avatrombopag and lusutrombopag for thrombocytopenia in people with chronic liver disease needing an elective procedure: a systematic review and cost-effectiveness analysis.[Health Technol Assess. 2020]Avatrombopag and lusutrombopag for thrombocytopenia in people with chronic liver disease needing an elective procedure: a systematic review and cost-effectiveness analysis.Armstrong N, Büyükkaramikli N, Penton H, Riemsma R, Wetzelaer P, Huertas Carrera V, Swift S, Drachen T, Raatz H, Ryder S, et al. Health Technol Assess. 2020 Oct; 24(51):1-220.
- Review Thrombopoietin Receptor Agonists (TPO-RAs): Drug Class Considerations for Pharmacists.[Drugs. 2021]Review Thrombopoietin Receptor Agonists (TPO-RAs): Drug Class Considerations for Pharmacists.Gilreath J, Lo M, Bubalo J. Drugs. 2021 Jul; 81(11):1285-1305. Epub 2021 Jun 23.
- Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study.[Br J Haematol. 2022]Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study.Al-Samkari H, Jiang D, Gernsheimer T, Liebman H, Lee S, Wojdyla M, Vredenburg M, Cuker A. Br J Haematol. 2022 May; 197(3):359-366. Epub 2022 Feb 18.
- Review Thrombopoietin Receptor Agonists: Eltrombopag and Romiplostim for the Treatment of Chronic Immune Thrombocytopenia Purpura.[Clin J Oncol Nurs. 2019]Review Thrombopoietin Receptor Agonists: Eltrombopag and Romiplostim for the Treatment of Chronic Immune Thrombocytopenia Purpura.Atkinson K. Clin J Oncol Nurs. 2019 Apr 1; 23(2):212-216.
- Thrombopoietin Receptor Agonists - LiverToxThrombopoietin Receptor Agonists - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...