NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor which is used in combination with diet and exercise in the therapy of type 2 diabetes, either alone or in combination with other oral hypoglycemic agents. Only rare isolated reports of liver injury due to sitagliptin have been published.
Background
Sitagliptin (sit" a glip' tin) is an inhibitor of dipeptidyl peptidase-4, which is the major enzyme responsible for the degradation of glucagon-like peptide-1 (GLP-1), an important gastrointestinal hormone (incretin) that increases glucose dependent insulin secretion by the pancreas. By prolonging the effect of GLP-1, sitagliptin increases insulin levels and lowers blood glucose and helps in glycemic control in patients with type 2 diabetes. Sitagliptin was approved for use in the United States in 2006 and was the first DPP-4 inhibitor introduced into clinical practice. The current indications are for management of glycemic control in type 2 diabetes used in combination with diet and exercise, with or without other oral hypoglycemic agents or insulin. Sitagliptin is available in tablets of 25, 50 and 100 mg under the brand name Januvia and in fixed combinations with metformin under the name Janumet. The typical dose of sitagliptin in adults is 100 mg once daily. Adverse reactions to sitagliptin are not common, but may include headache, nausea, arthralgia and rash. Hypoglycemia is uncommon with sitagliptin alone (<1%), but occurs in higher rates when it is combined with other oral hypoglycemic agents. Sitagliptin has also been linked to rare instances of bullous pemphigoid and acute pancreatitis that can be severe and even fatal.
Hepatotoxicity
Liver injury due to sitagliptin is rare. In large clinical trials, serum enzyme elevations were no more common with sitagliptin therapy (0.5%) than with placebo (0.4%), and no instances of clinically apparent liver injury were reported. Since licensure, instances of serum enzyme elevations attributed to sitagliptin have been reported to the FDA and the sponsor. A single case report of clinically apparent liver injury has been published, but in a patient who also had hepatitis C. The pattern of serum enzyme elevations was hepatocellular and peak serum bilirubin was 9.4 mg/dL, with a rapid recovery upon stopping sitagliptin. Immunoallergic features and autoantibodies were absent.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver injury during sitagliptin therapy is not known. The drug is mostly excreted unchanged and only a small proportion (~15%) is metabolized in the liver, largely by the cytochrome P450 system (CYP 3A4 and 2C8).
Outcome and Management
The instances of liver injury associated with the DPP-4 inhibitors have been self-limited and resolved rapidly upon stopping the medication. The similarity in chemical structure among the DPP-4 inhibitors suggests that there may be cross sensitivity to hepatic injury among the different agents, but this has not been reported. However, the other common antidiabetic medications in use should be tolerated without increased risk of liver injury.
References regarding the hepatotoxicity and safety of the DPP-4 inhibitors are given in the Overview section of DPP-4 Inhibitors (updated 03 January 2018).
Drug Class: Antidiabetic Agents, Incretin-Based Drugs
Other Drugs in the Subclass, Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: Alogliptin, Linagliptin, Saxagliptin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sitagliptin – Januvia®
DRUG CLASS
Antidiabetic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
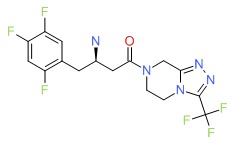
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sitagliptin | 486460-32-6 | C16-H15-F6-N5-O |
 |
- PubChem SubstanceRelated PubChem Substances
- Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin.[Diabetes Obes Metab. 2007]Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, Sitagliptin Study 035 Group. Diabetes Obes Metab. 2007 Sep; 9(5):733-45. Epub 2007 Jun 26.
- Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes.[J Clin Endocrinol Metab. 2006]Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes.Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, et al. J Clin Endocrinol Metab. 2006 Nov; 91(11):4612-9. Epub 2006 Aug 15.
- Review Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus.[Cochrane Database Syst Rev. 2008]Review Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Cochrane Database Syst Rev. 2008 Apr 16; 2008(2):CD006739. Epub 2008 Apr 16.
- Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea.[Transl Res. 2012]Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea.Aso Y, Ozeki N, Terasawa T, Naruse R, Hara K, Suetsugu M, Takebayashi K, Shibazaki M, Haruki K, Morita K, et al. Transl Res. 2012 Jan; 159(1):25-31. Epub 2011 Oct 17.
- Review Dipeptidyl peptidase-4 inhibitors in triple oral therapy regimens in patients with type 2 diabetes mellitus.[Curr Med Res Opin. 2015]Review Dipeptidyl peptidase-4 inhibitors in triple oral therapy regimens in patients with type 2 diabetes mellitus.Barnett AH, Charbonnel B, Moses RG, Kalra S. Curr Med Res Opin. 2015; 31(10):1919-31. Epub 2015 Sep 11.
- Sitagliptin - LiverToxSitagliptin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...