NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The dipeptidyl peptidase 4 (DPP-4) inhibitors are a family of diabetic agents that enhance glucagon-like peptide-1 (GLP-1) activity, a gastrointestinal hormone (incretin) that increases glucose dependent insulin secretion. The DPP-4 inhibitors decrease the degradation of GLP-1 and thereby increase endogenous circulating levels. The increase levels of GLP-1 in the circulation increase pancreatic insulin secretion and improve glycemic control in patients with type 2 diabetes. The DPP-4 inhibitors have only recently been introduced into clinical use and the full range of adverse events may not be fully known. However, in prelicensure clinical trials, sitagliptin, saxagliptin, linagliptin and alopgliptin, the first four DPP-4 inhibitors to receive FDA approval in the United States, were not associated with an increase in serum aminotransferase or alkaline phosphatase elevations above the rates found in controls, and no clinically apparent acute liver injury was reported. Since licensure, isolated case reports of liver injury have arisen but liver injury from these agents appears to be rare and generally mild.
As of 2018, the four DPP-4 inibitors that are available in the United States include (with brand names and year of approval): sitagliptin (Januvia, 2006), saxagliptin (Onglyza, 2009), linagliptin (Tradjenta, 2011) and alogliptin (Nesina, 2013). These four DPP-4 inhibitors are discussed individually in LiverTox, but references to their safety and potential hepatotoxicity are given together after this introductory section. DDP-4 inhibitors in clinical use in other countries include vildagliptin, gemigliptin, anagliptin, omarigliptin, evogliptin, gosogliptin and teneligliptin.
The four DDP-4 inhibitors have been linked to rare instances of self-limited, cholestatic or mixed liver injury that generally arises 1 to 4 weeks after initiation of therapy and resolves without residual injury within 1 to 3 months.
Drug Class: Antidiabetic Agents
Drugs in the Subclass Incretin-Based Drugs, DPP-4 Inhibitors: Alogliptin, Linagliptin, Saxagliptin, Sitagliptin
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Alogliptin | 850649-62-6 | C18-H21-N5-O2.C7-H6-O2 |
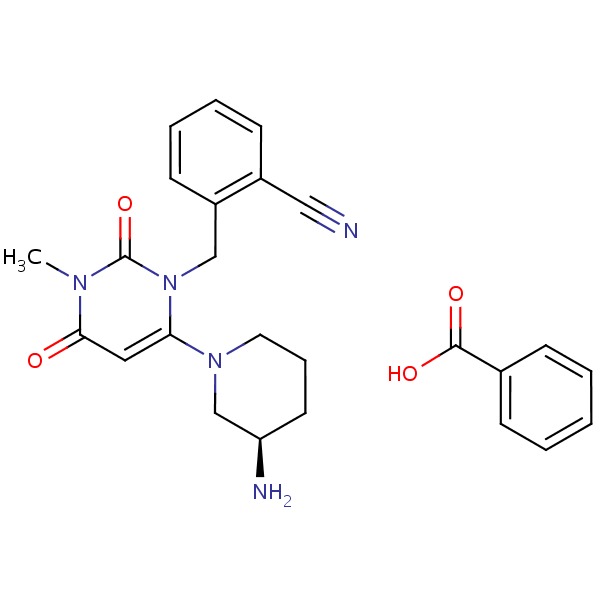 |
| Linagliptin | 668270-12-0 | C25-H28-N8-O2 |
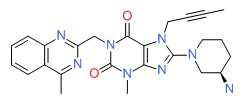 |
| Saxagliptin | 945667-22-1 | C18-H25-N3-O2.H2O |
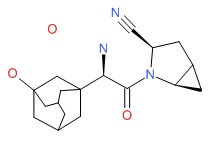 |
| Sitagliptin | 486460-32-6 | C16-H15-F6-N5-O |
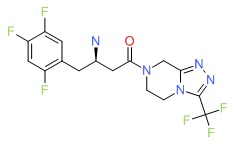 |
ANNOTATED BIBLIOGRAPHY
References updated: 03 January 2018
- Zimmerman HJ. Oral hypoglycemic agents and other diabetes therapy. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 575-9.(Textbook of hepatotoxicity published in 1999 and before the availability of sitagliptin and other DPP-4 inhibitors).
- De Marzio DH, Navarro VJ. Antidiabetic drugs. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 528-32. (Review of hepatotoxicity of drugs for diabetes.mentions that there have been two case reports of liver injury attributed to sitaglitin but none for linagliptin or saxagliptin).
- Powers AC, D'Alessio D. Therapy of diabetes. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1248-67.(Textbook of pharmacology and therapeutics).
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298: 194-206. [PubMed: 17622601](Systematic review of 29 controlled trials of incretin therapy [exenatide, liraglutide, sitagliptin and vildagliptin], concluding that they have "modest efficacy" but were well tolerated, with few side effects occurring more frequently than in controls and no mention of ALT levels or hepatotoxicity).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver injury in the US collected from 2004 to 2008, none were attributed DPP-4 inhibitors which were rarely used during the period of this study).
- Gross BN, Cross LB, Foard J, Wood Y. Elevated hepatic enzymes potentially associated with sitagliptin. Ann Pharmacother 2010; 44: 394-5. [PubMed: 20103614](58 year old man with suspected nonalcoholic fatty liver disease developed asymptomatic liver test elevations 1 month after starting sitagliptin [ALT 127 U/L, bilirubin and Alk P normal], which fell upon withdrawal [to 90 U/L after 1 and 62 U/L after 6 months]).
- Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 2010; 86: 44-57. [PubMed: 20616619](Review of safety and efficacy of sitagliptin in 11 controlled trials; no mention of ALT levels or hepatotoxicity).
- Lam S, Saad M. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for type 2 diabetes. Cardiol Rev 2010; 18: 213-7. [PubMed: 20539105](Review of mechanism of action, pharmacology, clinical efficacy and safety of saxagliptin; side effects are usually mild and similar in frequency to placebo; no mention of ALT levels or hepatotoxicity).
- Seck T, Nauck M, Sheng D, Sunga S, Davies MJ, Stein PP, Kaufman KD, Amatruda JM; Sitagliptin Study 024 Group. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract 2010; 64: 562-76. [PubMed: 20456211](Controlled trial comparing addition of sitagliptin vs glipizide to metformin in 1172 patients with diabetes with 2 year follow up; there were no differences in rates of ALT elevations between the two groups, but rates were not given).
- Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, et al. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord 2010; 10: 7. [PMC free article: PMC3161395] [PubMed: 20412573](Pooled analysis of safety of sitagliptin in 10,245 patients [19 studies] with diabetes treated for 12 weeks to 2 years; ALT above 3 times ULN occurred in 0.5% [1.5 per 100 patient-years] of sitagliptin vs 0.4% [1.4 per 100] on comparative agents; no mention of clinically apparent liver injury).
- Neumiller JJ, Wood L, Campbell RK. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy 2010; 30: 463-84. [PubMed: 20411998](Systematic review of literature on safety and efficacy of DPP-4 inhibitors; rates of serum enzyme elevations were similar in DPP-4 vs control treated patients, although enzyme elevations have been listed as an adverse event based on postmarketing reporting).
- Toyoda-Akui M, Yokomori H, Kaneko F, Shimizu Y, Takeuchi H, Tahara K, Motoori T, et al. A case of drug-induced hepatic injury associated with sitagliptin. Intern Med 2011; 50: 1015-20. [PubMed: 21532224](58 year old man developed jaundice 1 month after starting sitagliptin [bilirubin 11.8 rising to 20.5 mg/dL, ALT 1653 U/L, Alk P 814 U/L, HCV RNA and anti-HCV positive], resolving within 1-2 months; did not exclude acute hepatitis C).
- Linagliptin (Tradjenta): a new DPP-4 inhibitor for type 2 diabetes. Med Lett Drugs Ther 2011; 53 (1367): 49-50. [PubMed: 21701440](Concise review of efficacy and safety of linagliptin shortly after its approval; no mention of hepatotoxicity, but 8 of 487 patients on linagliptin developed pancreatitis, compared to none of 1183 on placebo).
- Scott LJ. Linagliptin: in type 2 diabetes mellitus. Drugs 2011; 71: 611-24. (Summary of structure, pharmacology, clinical efficacy and tolerability of linagliptin, a new DPP-4 inhibitor; tolerab. [PubMed: 21443284]ility was similar to other groups including placebo and "there were no clinically significant changes in laboratory parameters").
- Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, Woerle HJ. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011; 13: 65-74. [PubMed: 21114605](Controlled trial of 24 week course of linagliptin vs placebo in 701 patients; "The analysis of laboratory variables...did not reveal any clinically significant findings").
- Arase Y, Suzuki F, Kobayashi M, Suzuki Y, Kawamura Y, Matsumoto N, Akuta N, et al. Efficacy and safety in sitagliptin therapy for diabetes complicated by chronic liver disease caused by hepatitis C virus. Hepatol Res 2011; 41: 524-9. [PubMed: 21435130](Controlled trial of sitagliptin vs placebo in 32 patients with diabetes and chronic hepatitis C showed no change in mean ALT levels during 48 weeks of treatment in either group).
- Drugs for type 2 diabetes. Treat Guidel Med Lett 2011; 9 (108): 47-54. [PubMed: 21778966](Concise overview on the use of medications in diabetes).
- Mikhail N. Safety of dipeptidyl peptidase 4 inhibitors for treatment of type 2 diabetes. Curr Drug Saf 2011; 6: 304-9. [PubMed: 22424537](Review of the safety of the DPP-4 inhibitors, discussed pancreatitis, but not liver injury).
- Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin 2011; 27 Suppl 3:57-64. [PubMed: 22106978](Metaanalysis of 53 trials enrolling 30,312 patients on DPP-4 inhibitors and 13,569 on comparator agents, found no differences in rates of cancer, pancreatitis or major cardiovascular events; no mention of liver injury).
- Richard KR, Shelburne JS, Kirk JK. Tolerability of dipeptidyl peptidase-4 inhibitors: a review. Clin Ther 2011; 33: 1609-29. [PubMed: 22071236](Systematic review of literature on tolerability of DPP inhibitors found 45 clinical trials of 1 to 104 weeks of therapy; common side effects were headache, gastrointestinal symptoms and pain; no mention of ALT elevations or liver injury).
- Jeong KH, Yoo BK. The efficacy and safety of liraglutide. Int J Clin Pharm 2011; 33: 740-9. [PubMed: 21952951](Systematic review of literature on liraglutide, a long acting GLP analogue, found discontinuation rates because of adverse events in 2-12% of liraglutide vs 2-13% of comparator agents; no mention of hepatic toxicity).
- Neumiller JJ. Pharmacology, efficacy, and safety of linagliptin for the treatment of type 2 diabetes mellitus. Ann Pharmacother 2012; 46: 358-67. [PubMed: 22318932](Review of the pharmacology, efficacy and safety of linagliptin; pancreatitis occurred in 8 of 4687 linagliptin recipients compared to none of 1183 controls; no mention of liver injury and linapliptin is said to have low propensity for drug interactions).
- Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother 2012; 46: 1453-69. [PubMed: 23136353](Systematic review efficacy and safety of DPP-4 inhibitors based upon published controlled trials; side effects were similar in recipients of DPP-4 inhibitors as placebo controls; no mention of ALT elevations or hepatotoxicity).
- Bergenstal RM, Forti A, Chiasson JL, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T-emerge 4 trial). Diabetes Ther 2012; 3: 13. [PMC free article: PMC3508113] [PubMed: 23138449](Controlled trial of taspoglutide vs sitagliptin in 666 patients with diabetes for 24 weeks; no mention of ALT elevations or hepatotoxicity).
- Gooßen K, Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab 2012; 14 (12): 1061-72. [PubMed: 22519906](Systematic review of controlled trials of DPP-4 inhibitors focusing on long term safety found no evidence of an increase rate of infections; no mention of ALT elevations or hepatotoxicity).
- Lo Re V 3rd, Haynes K, Ming EE, Wood Ives J, Horne LN, Fortier K, Carbonari DM, et al. Safety of saxagliptin: rationale for and design of a series of postmarketing observational studies. Pharmacoepidemiol Drug Saf 2012; 21: 1202-15. [PubMed: 22763953](Design of large, retrospective postmarketing studies of saxagliptin aimed at assessing rare adverse reactions including liver injury).
- Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab 2012; 14: 927-36. [PubMed: 22583697](Among 288 patients with type 2 diabetes on metformin who were treated with two doses of alogliptin for up to 52 weeks, ALT elevations occurred in 1.4 and 3.4%, but none were considered "clinically significant").
- Jarvis CI, Cabrera A, Charron D. Alogliptin: a new dipeptidyl peptidase-4 inhibitor for type 2 diabetes mellitus. Ann Pharmacother 2013; 47: 1532-9. [PubMed: 24285765](Systematic review of the efficacy and safety of alogliptin mentions that in multiple controlled studies, "no clinically relevant changes in laboratory assessments" were noted but that, since licensure, cases of pancreatitis, hypersensitivity reactions and fatal and non-fatal hepatic failure have been reported to the sponsor).
- Alogliptin (Nesina) for type 2 diabetes. Med Lett Drugs Ther 2013; 55 (1417): 41-3. [PubMed: 23715128](Concise summary of mechanism of action, efficacy and safety of alogliptin shortly after its approval for use in the US mentions that reported severe adverse effects include acute pancreatitis, hypoglycemia, hypersensitivity reactions, Stevens Johnson syndrome and fatal hepatic failure).
- Drugs for type 2 diabetes. Treat Guidel Med Lett 2014; 12 (139): 17-24. [PubMed: 24566424](Concise summary of efficacy and safety of drugs for type 2 diabetes and guidelines on their use; listed side effects of the four DPP-4 inhibitors approved for use in the US include hypersensitivity reactions, acute pancreatitis and fatal hepatic failure).
- Seino Y, Yabe D. Alogliptin benzoate for the treatment of type 2 diabetes. Expert Opin Pharmacother 2014; 15: 851-63. [PubMed: 24646052](Review of the structure, mechansim of action, pharmacokinetics, efficacy and safety of alogliptin mentions that it is generally well tolerated and that in 13 controlled trials, adverse events were similar in all treatment arms; no specific discussion of hepatotoxicity or ALT levels).
- Kutoh E. Probable linagliptin-induced liver toxicity: A case report. Diabetes Metab 2014; 40: 82-4. [PubMed: 24378344](58 year old woman developed fatigue and jaundice 4 weeks after starting linagliptin [peak bilirubin 2.1 mg/dL, ALT 1006, Alk P 2.1 times ULN], with rapid improvement in symptoms, but slow resolution of abnormal enzymes upon stopping).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a DPP-4 inhibitor or other antidiabetic medication).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, but none of the cases were attributed to a DPP-4 inhibitor or other anti-diabetic medication).
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, et al.; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327-35. [PubMed: 23992602](Among 5380 patients with type 2 diabetes and coronary artery disese treated with alogliptin or placebo for a median of 18 months, rates of death and cardiovascular and cerebrovascular events were similar in the two groups, as were rates of serum ALT elevations [2.4% vs 1.7%] and AST elevations [1.8% vs 1.6%] above 3 times ULN).
- Hirshberg B, Parker A, Edelberg H, Donovan M, Iqbal N. Safety of saxagliptin: events of special interest in 9156 patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2014; 30: 556-69. [PubMed: 24376173](Pooled analysis of 20 controlled trials of saxagliptin in 9156 patients with type 2 diabetes found similar rates of severe adverse events and deaths with saxagliptin vs placebo or standard care treatment and similar rates of ALT or AST elevations [1.5% vs 1.3% of patients had values that rose above 3 times ULN]).
- Asakawa M, Mitsui H, Akihisa M, Sekine T, Niitsu Y, Kobayashi A, Miyake A, et al. Efficacy and safety of sitagliptin for the treatment of diabetes mellitus complicated by chronic liver injury. Springerplus. 2015; 4: 346. [PMC free article: PMC4501339] [PubMed: 26191473](Among 122 patients with type 2 diabetes and chronic liver injury treated with sitagliptin for an average of one year, there was a significant decrease in mean HbA1c levels, but no worsening of ALT [decreasing: 75 U/L to 66 U/L] or AST [no changing: 56 U/L to 57 U/L]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 4 cases [0.5%] were at least possibly attributed to antidiabetic medications including 1 to sitagliptin).
- Lo Re V, Carbonari DM, Saine ME, Newcomb CW, Roy JA, Liu Q, Wu Q, et al. Postauthorization safety study of the DPP-4 inhibitor saxagliptin: a large-scale multinational family of cohort studies of five outcomes. BMJ Open Diabetes Res Care 2017; 5: e000400. [PMC free article: PMC5574452] [PubMed: 28878934](Analysis of patients with diabetes from 4 large health care networks between 2009 and 2014 for adverse events following initiation of therapy with saxagliptin compared to use of other antidiabetic agents found no increased rates of acute liver or kidney failure or severe hypersensitivity reactions).
- Rehman MB, Tudrej BV, Soustre J, Buisson M, Archambault P, Pouchain D, Vaillant-Roussel H, Gueyffier F, et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: Meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab 2017; 43: 48-58. [PubMed: 27745828](Among 36 controlled trials of DPP-4 inhibitors involving 54,664 patients with type 2 diabetes, there was no increase in rates of mortality, myocardial infarction, stroke or renal failure, but slight increase in rates of acute pancreatitis and heart failure).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review An update on DPP-4 inhibitors in the management of type 2 diabetes.[Expert Opin Emerg Drugs. 2016]Review An update on DPP-4 inhibitors in the management of type 2 diabetes.Cahn A, Cernea S, Raz I. Expert Opin Emerg Drugs. 2016 Dec; 21(4):409-419. Epub 2016 Nov 18.
- Bullous pemphigoid and dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized controlled trials.[Endocrine. 2020]Bullous pemphigoid and dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized controlled trials.Silverii GA, Dicembrini I, Nreu B, Montereggi C, Mannucci E, Monami M. Endocrine. 2020 Sep; 69(3):504-507. Epub 2020 Mar 31.
- Review Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus.[Clin Exp Pharmacol Physiol. 2015]Review Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus.Chen XW, He ZX, Zhou ZW, Yang T, Zhang X, Yang YX, Duan W, Zhou SF. Clin Exp Pharmacol Physiol. 2015 Oct; 42(10):999-1024.
- Review Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas.[Diabetes Obes Metab. 2016]Review Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas.Deacon CF, Lebovitz HE. Diabetes Obes Metab. 2016 Apr; 18(4):333-47. Epub 2016 Jan 8.
- Review Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors.[J Am Pharm Assoc (2003). 2009]Review Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors.Neumiller JJ. J Am Pharm Assoc (2003). 2009 Sep-Oct; 49 Suppl 1:S16-29.
- Dipeptidyl Peptidase-4 Inhibitors - LiverToxDipeptidyl Peptidase-4 Inhibitors - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...