NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sildenafil functions as a selective and competitive inhibitor of type 5 phosphodiesterases (PDE5) on smooth muscle cells in the penis and pulmonary vasculature, and is used extensively for erectile dysfunction and less commonly for pulmonary hypertension. Sildenafil has been associated with rare instances of clinically apparent liver injury.
Background
Sildenafil (sil den' a fil) is an inhibitor of type 5 phosphodiesterase (PDE5), the enzyme isoform that is found predominantly in the penis and lungs. Inhibition of PDE5 leads to prolongation of activation of cyclic guanosine monophosphate (cGMP), a major mediator of the vasodilatory action of nitric oxide (NO). Inhibition of PDE5 leads to prolongation of smooth muscle relaxation in the corpus cavernosum of the penis (improving erections) and in the pulmonary vasculature (decreasing pulmonary artery pressure). Sildenafil was approved for use in the therapy of erectile dysfunction in the United States in 1998 and is available generically and under the brand name Viagra in tablets of 25, 50 and 100 mg. Worldwide, more than 20 million men have used sildenafil for erectile dysfunction. The recommended dose is 50 mg as a single dose approximately one hour before sexual activity, with dose increase or decrease based upon tolerance and effectiveness with a recommended maximum frequency of once daily and maximum amount of 100 mg. Sildenafil is also approved for use in pulmonary hypertension as tablets of 20 mg generically and under the brand name Revatio. The recommended dosage for pulmonary hypertension is 20 mg three times daily. An intravenous formulation of sidenafil is also available in 10 mg vials for more emergent treatment of pulmonary artery hypertension, the recommended dosage being 10 mg intravenously three times daily. Common side effects include dizziness, headache, flushing, hypotension, rhinitis and dyspepsia. Rare, but potentially serious adverse events include vision and hearing loss, hypotension, cardiovascular events and priapism.
Hepatotoxicity
There have been at least 5 reports of acute liver injury attibuted to sildenafil use, but no instances of acute hepatic failure. The latency in most reports has been unclear because of the intermittent and sometimes unacknowledged use of sildenafil, but appears to be within 1 to 8 weeks. The pattern of serum enzyme elevations has ranged from hepatocellular to cholestatic, sometimes evolving from one to the other. The most convincing cases have been a mild cholestatic or "mixed" hepatitis arising within 1 to 3 months of starting sildenafil. Immunoallergic features and autoantibodies were not observed. Cases of acute onset with high serum aminotransferase levels have been reported after use of sildenafil that have some characteristics of ischemic injury. In other instances, the pattern of injury suggested anabolic steroid use. In two cases, re-exposure did not result in recurrence. Thus, the hepatotoxicity of sildenafil is not completely convincing and must be quite rare, if it occurs at all.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of hepatic injury due to sildenafil is still unknown, but it is metabolized by the P450 system (CYP 3A4), and idiosyncratic production of a toxic intermediate may underlie cases of hepatotoxicity from the agent. In some instances, ischemic injury to the liver may be responsible for acute, self-limited injury due to hypotension or an acute cardiac event triggered by sildenafil induced vasodilation or sexual activity, particularly if they are taken with nitrates.
Outcome and Management
Cases of acute liver injury from sildenafil have been self-limited, and full recovery occurred in all reported cases without residual liver injury or vanishing bile duct syndrome. Rechallenge has not been reported to lead to recurrence, and cross reactivity with other PDE5 inhibitors has not been reported. Nevertheless, switching to another PDE5 inhibitor after clinically apparent liver injury should be done with caution.
References to sidenafil induced liver injury are provided in the Overview section on PDE5 Inhibitors.
Drug Class: PDE5 Inhibitors
Other Drugs in the Class: Avanafil, Tadalafil, Vardenafil
CASE REPORT
Case 1. Acute cholestatic hepatitis caused by sildenafil.
[Modified from: Wolfhagen FH, Vermeulen HG, de Man RA, Lesterhuis W. Initially obscure hepatotoxicity attributed to sildenafil. Eur J Gastroenterol Hepatol 2008; 20: 710-2. PubMed Citation]
A 59 year old man developed fatigue, nausea and jaundice that persisted for several weeks. When initially seen, blood tests showed serum bilirubin of 5.2 mg/dL with marked elevation in aminotransferase levels (ALT 1665 U/L, AST 1077 U/L), but minimal increases in alkaline phosphatase (173 U/L) (Table). Because of persistence of jaundice and pruritus, he was admitted for evaluation. Physical examination revealed jaundice and hepatic tenderness. Serum bilirubin rose to 19 mg/dL. Tests for hepatitis A, B and C and for CMV and EBV were negative as were autoantibodies. Ultrasound showed no evidence of biliary tract disease. A liver biopsy showed severe intrahepatic cholestasis and eosinophils in portal areas suggestive of drug induced liver injury. At this point, the patient admitted to taking sildenafil in doses of 50 mg several times per week for the 3 months before onset of illness. He took no other medications and drank alcohol sparingly. Over the ensuing 1 to 2 months, he recovered and all laboratory tests returned to normal.
Key Points
| Medication: | Sildenafil (50 mg daily, several times weekly) |
| Pattern: | Hepatocellular evolving into cholestatic (R=~40 → 2) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | ~3 months |
| Recovery: | ~3 months |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Sildenafil used several times per week for 3 months before onset of symptoms | |||||
| 3 months | 0 | 1665 | 173 | 5.2 | Outpatient evaluation |
| 1 week | 120 | 140 | 7.9 | ||
| 4 months | 4 weeks | 59 | 150 | 15.0 | Hospitalization |
| 7 weeks | 70 | 210 | 17.8 | Severe pruritus | |
| 5 months | 8 weeks | 65 | 145 | 19.0 | Liver biopsy |
| 9 weeks | 40 | 125 | 13.5 | ||
| 6 months | 10 weeks | 35 | 105 | 5.6 | |
| 14 weeks | 30 | 90 | 1.5 | ||
| 7 months | 4 months | 20 | 75 | 0.7 | |
| 8 months | 5 months | 25 | 75 | 0.7 | Asymptomatic |
| Normal Values | <40 | <100 | <1.2 | ||
*Selected values estimated from Figure 1.
Comment
The clinical course was typical for a cholestatic drug induced liver injury and, in the absence of evidence for biliary disease and lack of other medication exposure, sildenafil is certainly suspect. This report is perhaps the most convincing example of drug induced liver injury from sildenafil. The clinical course was also compatible with liver injury from anabolic steroids, which are often not mentioned in a routine history. Rechallenge was not performed because of the severity and duration of the hepatic injury; an appropriate decision.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sildenafil – Generic, Viagra®, Revatio®
DRUG CLASS
PDE5 Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sildenafil | 139755-83-2 | C22-H30-N6-O4-S |
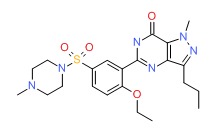 |
- PubChem SubstanceRelated PubChem Substances
- Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro.[Am J Cardiol. 1999]Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro.Wallis RM, Corbin JD, Francis SH, Ellis P. Am J Cardiol. 1999 Mar 4; 83(5A):3C-12C.
- Review Phosphodiesterase Type 5 (PDE5) Inhibitors.[LiverTox: Clinical and Researc...]Review Phosphodiesterase Type 5 (PDE5) Inhibitors.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction.[Urology. 2002]Review Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction.Corbin JD, Francis SH, Webb DJ. Urology. 2002 Sep; 60(2 Suppl 2):4-11.
- Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction.[J Androl. 2004]Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction.De Young L, Yu D, Bateman RM, Brock GB. J Androl. 2004 Sep-Oct; 25(5):830-6.
- In vivo analysis of chronic phosphodiesterase-5 inhibition with sildenafil in penile erectile tissues: no tachyphylaxis effect.[J Urol. 2005]In vivo analysis of chronic phosphodiesterase-5 inhibition with sildenafil in penile erectile tissues: no tachyphylaxis effect.Musicki B, Champion HC, Becker RE, Kramer MF, Liu T, Sezen SF, Burnett AL. J Urol. 2005 Oct; 174(4 Pt 1):1493-6.
- Sildenafil - LiverToxSildenafil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...