NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Avanafil is a selective inhibitor of phosphodiesterase type 5 (PDE5) and is used as therapy of erectile dysfunction. Avanafil is a relatively new medication and has yet to be linked to instances of serum enzyme elevations or with clinically apparent acute liver injury.
Background
Avanafil (a van' a fil) is a selective inhibitor of phosphodiesterase type 5 (PDE5) which mediates the breakdown of cyclic guanosine monophosphate (cGMP), inducing smooth muscle relaxation in the corpus cavernosum of the penis and in the pulmonary vasculature where this specific phosphodiesterase is found. Avanafil is effective in prolonging erection and was approved for use in the United States in 2012. Avanafil is available in tablets of 50, 100 and 200 mg under the brand name of Stendra. The recommended dose is 100 mg as a single dose as needed one-half hour before sexual activity. The dose can be increased or decreased based upon effect and tolerance with a recommended maximum frequency of once daily and maximum dosage of 200 mg. Common side effects include dizziness, headache, flushing, hypotension, rhinitis and dyspepsia. Rare, but potentially serious adverse events include vision and hearing loss, hypotension, cardiovascular events and priapism.
Hepatotoxicity
Avanafil has had limited general use, but in premarketing studies it was not associated with cases of clinically apparent liver injury and serum enzyme elevations were not reported. The related PDE5 inhibitors, sildenafil and tadalafil, have been linked to isolated, rare instances of acute liver injury and jaundice. The latency to onset ranged from a few days to 3 months and the pattern of injury was usually cholestatic. Autoimmune and immunoallergic features were not observed and all cases were self-limited without residual injury or acute liver failure. Whether avanafil can cause a similar form of acute liver injury is not yet known.
Likelihood score: E* (unproved but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
While avanafil has not been associated with hepatotoxicity, its potential for causing hypotension and use in patients with cardiac disease may lead to instances of acute ischemic liver injury. Avanafil, like the other PDE5 inhibitors, is metabolized in the liver via the cytochrome P450 system (CYP 3A4).
Outcome and Management
There is no known cross sensitivity between avanafil and the other PDE5 inhibitors currently in use in the United States. However, switching to another PDE5 inhibitor after an episode of clinically apparent liver injury should be done with caution.
References to the safety and potential hepatotoxicity of avanafil are provided in the Overview section on PDE5 Inhibitors.
Drug Class: PDE5 Inhibitors
Other Drugs in the Class: Sildenafil, Tadalafil, Vardenafil
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Avanafil – Stendra ®
DRUG CLASS
PDE5 Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
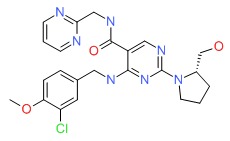
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Avanafil | 330784-47-9 | C23-H26-Cl-N7-O3 |
 |
- Review Avanafil for erectile dysfunction.[Ann Pharmacother. 2013]Review Avanafil for erectile dysfunction.Kyle JA, Brown DA, Hill JK. Ann Pharmacother. 2013 Oct; 47(10):1312-20. Epub 2013 Sep 27.
- Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: implications for clinical safety and improved tolerability.[J Sex Med. 2012]Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: implications for clinical safety and improved tolerability.Wang R, Burnett AL, Heller WH, Omori K, Kotera J, Kikkawa K, Yee S, Day WW, DiDonato K, Peterson CA. J Sex Med. 2012 Aug; 9(8):2122-9. Epub 2012 Jul 3.
- Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double-blind, placebo-controlled trial.[BJU Int. 2012]Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double-blind, placebo-controlled trial.Zhao C, Kim SW, Yang DY, Kim JJ, Park NC, Lee SW, Paick JS, Ahn TY, Moon KH, Chung WS, et al. BJU Int. 2012 Dec; 110(11):1801-6. Epub 2012 Mar 27.
- Review Avanafil for treatment of erectile dysfunction: review of its potential.[Vasc Health Risk Manag. 2012]Review Avanafil for treatment of erectile dysfunction: review of its potential.Burke RM, Evans JD. Vasc Health Risk Manag. 2012; 8:517-23. Epub 2012 Aug 29.
- Review Avanafil for the treatment of erectile dysfunction. An updated review.[Arch Esp Urol. 2014]Review Avanafil for the treatment of erectile dysfunction. An updated review.Egui-Rojo MA, Moncada-Iribarren I, Carballido-Rodríguez J, Martínez-Salamanca JI. Arch Esp Urol. 2014 Dec; 67(10):839-47.
- Avanafil - LiverToxAvanafil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...