NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Roflumilast is a selective inhibitor of phosphodiesterase-4 (PDE-4) that has unique antiinflammatory activity and is used to treat and prevent exacerbations of chronic obstructive pulmonary disease (COPD). Roflumilast has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury.
Background
Roflumilast (roe flue' mi last) is a long acting inhibitor of the enzyme, phosphodiesterase-4 (PDE-4), an oral antiinflammatory agent that is used to treat chronic obstructive pulmonary disease (COPD). PDE-4 is responsible for the metabolism of cyclic adenosine monophosphate (cAMP) and its inhibition results in increased intracellular concentrations of cAMP, an important mediator of inflammatory pathways. Roflumilast has been shown to decrease pro-inflammatory cytokines and reduce inflammatory responses in chronic pulmonary conditions including chronic bronchitis. In large controlled trials, roflumilast therapy was associated with a decrease in the numbers of severe, acute exacerbations of COPD and a modest improvement in pulmonary function test results. Roflumilast was approved is 2011 for use in patients with COPD to prevent acute exacerbations of chronic bronchitis. It is currently available in tablets of 500 µg under the brand name Daliresp and the recommended dose in 500 µg once daily. Side effects are generally mild and include diarrhea, nausea, vomiting, decreased appetite, weight loss, headache, dizziness, insomnia and back and joint pain. Rare, but potentially severe adverse reactions include depression, suicidality and hypersensitivity reactions, including angioedema.
Hepatotoxicity
In preregistration studies, roflumilast was not associated with serum enzyme elevations or with episodes of clinically apparent liver injury. Since approval of roflumilast, there have been no published reports of hepatotoxicity, and the product label does not mention liver injury as an adverse event.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Liver Injury
The mechanism by which roflumilast might cause serum aminotransferase elevations or liver injury is not known. Its lack of hepatotoxicity may relate to the very low doses used (<1 mg daily). Roflumilast is metabolized by the hepatic cytochrome P450 system (predominately CYP 3A4 and 1A2) and is susceptible to drug-drug interactions.
Drug Class: Pulmonary Disease Agents, Chronic Obstructive Pulmonary Disease Agents
Other Drugs in the Subclass: Anticholinergic Agents – Ipratropium, Tiotropium, Aclidinium; Beta-2 Adrenergic Agonists – Albuterol, Formoterol, Salmeterol, Terbutaline; Corticosteroids – Budesonide, Fluticasone; Theophylline
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Roflumilast – Daliresp®
DRUG CLASS
Pulmonary Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
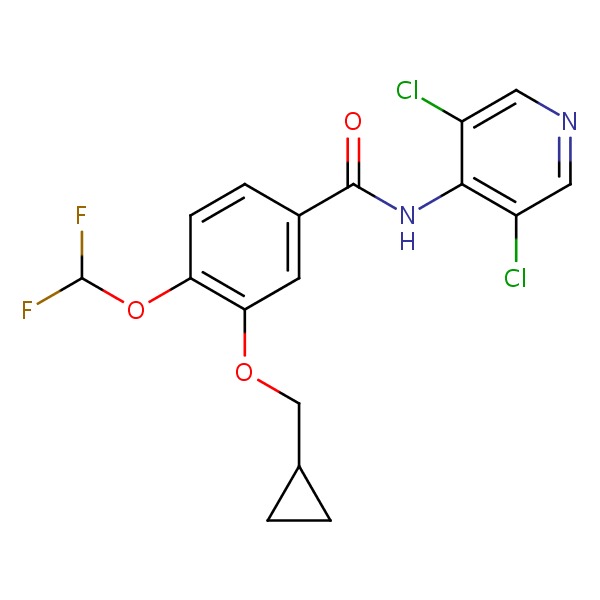
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Roflumilast | 162401-32-3 | C17-H14-Cl2-F2-N2-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 June 2018
Abbreviations used: COPD, chronic obstructive pulmonary disease; PDE-4, phosphodiesterase 4; cAMP, cyclic adenosine monophosphate.
- Zimmerman HJ. Respiratory supportive drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity. The adverse effects of drugs and other chemicals upon the liver. 2nd edition. Philadelphia. Lippincott, 1999. pp 717.(Textbook of drug induced liver injury published in 1999; does not discuss roflumilast).
- Barnes PJ. Pulmonary Pharmacology. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1031-65.(Textbook of pharmacology and therapeutics).
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 2005; 365 (9454): 167-75. [PubMed: 15639300](Review of the mechanism of action of PDE-4 inhibitors that act by increasing intracellular cAMP levels, which have antiinflammatory actions, decreasing chemotaxis, cell proliferation and production and release of cytokines; in clinical studies, roflumilast led to a reduction in the number of clinical exacerbations and “did not lead to any clinically significant changes in … laboratory test results”).
- Bateman ED, Izquierdo JL, Harnest U, Hofbauer P, Magyar P, Schmid-Wirlitsch C, Leichtl S, et al. Efficacy and safety of roflumilast in the treatment of asthma. Ann Allergy Asthma Immunol 2006; 96: 679-86. [PubMed: 16729780](Among 693 patients with asthma treated with roflumilast [100, 250 or 500 µg daily] for 12 weeks, pulmonary function tests improved and side effects were most frequent with the highest dose, while “changes in transaminase levels were not considered clinically significant”).
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176: 154-61. [PubMed: 17463412](Among 1513 patients with severe COPD treated with roflumilast [500 µg daily] or placebo for up to one year, pulmonary tests improved somewhat, but rates of acute exacerbation were similar in the two groups and adverse events of roflumilast were headache, diarrhea and nausea while “routine laboratory tests…did not show any clinically significant changes”).
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, Rabe KF; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374 (9691): 695-703. [PubMed: 19716961](In a pooled analysis of two placebo controlled trials in 2,131 patients with moderate-to-severe COPD, roflumilast therapy was associated with a modest improvement in pulmonary function test results but variable effects on symptoms, while side effects included decreased appetite, weight loss, nausea, diarrhea and headache and “routine laboratory tests…did not show any clinically significant changes”).
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374 (9691): 685-94. [PubMed: 19716960](In a pooled analysis of two placebo controlled trials in 3091 patients with symptomatic COPD, roflumilast was associated with modest improvements in pulmonary function tests and with fewer disease relapses, while side effects included decreased appetite, weight loss, nausea, diarrhea and headache; no mention of ALT changes or liver related adverse events).
- Roflumilast (Daliresp) for COPD. Med Lett Drugs Ther 2011; 53 (1369): 59-60. [PubMed: 21778965](Concise review of the mechanism of action, pharmacology, clinical efficacy, safety, drug interactions and costs of roflumilast shortly after its approval in the US, mentions side effects of diarrhea, nausea and weight loss, but does not mention ALT elevations or hepatotoxicity).
- Drugs for asthma and COPD. Treat Guidel Med Lett 2013; 11 (132): 75-86; [PubMed: 23896773](Concise discussion of therapies for COPD, including smoking cessation, short and long acting bronchodilators, theophylline, corticosteroids and other therapies such as roflumilast; no mention of ALT elevations or clinically apparent liver injury with any of the agents).
- Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, Anzueto A, Alagappan VK, et al. Effect of Roflumilast and Inhaled Corticosteroid/Long-Acting β2-Agonist on Chronic Obstructive Pulmonary Disease Exacerbations (RE(2)SPOND). A Randomized Clinical Trial. Am J Respir Crit Care Med 2016; 194: 559-67. [PubMed: 27585384](Among 2,354 patients with COPD at risk of acute exacerbations [and receiving inhaled corticosteroids and long acting beta agonists] treated with roflumilast [500 µg daily] or placebo for 52 weeks, acute exacerbations were reduced minimally [8.5%] and side effects of weight loss, diarrhea and nausea were more frequent with roflumilast; no mention of ALT elevations or liver related serious adverse events).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to roflumilast).
- Luo J, Yang L, Yang J, Yang D, Liu BC, Liu D, Liang BM, et al. Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: a systematic review and meta-analysis. Respirology 2018; 23: 467-77. [PubMed: 29502338](Systematic review of the literature on efficacy and safety of roflumilast in patients with asthma concluded that it was well tolerated “no clinically relevant alterations in …. routine laboratory tests including haematology, biochemistry and urinalysis were observed”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease.[Cochrane Database Syst Rev. 2020]Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease.Janjua S, Fortescue R, Poole P. Cochrane Database Syst Rev. 2020 May 1; 5(5):CD002309. Epub 2020 May 1.
- Review The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.[Pulm Pharmacol Ther. 2010]Review The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, Schudt C, Tenor H. Pulm Pharmacol Ther. 2010 Aug; 23(4):235-56. Epub 2010 Apr 7.
- Review Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD.[Drug Des Devel Ther. 2010]Review Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD.Giembycz MA, Field SK. Drug Des Devel Ther. 2010 Jul 21; 4:147-58. Epub 2010 Jul 21.
- Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol.[Int J Chron Obstruct Pulmon Di...]Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol.Calverley PM, Martinez FJ, Fabbri LM, Goehring UM, Rabe KF. Int J Chron Obstruct Pulmon Dis. 2012; 7:375-82. Epub 2012 Jun 20.
- Review Roflumilast in the management of chronic obstructive pulmonary disease.[Am J Health Syst Pharm. 2013]Review Roflumilast in the management of chronic obstructive pulmonary disease.Lipari M, Benipal H, Kale-Pradhan P. Am J Health Syst Pharm. 2013 Dec 1; 70(23):2087-95.
- Roflumilast - LiverToxRoflumilast - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...