NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Riociguat is a stimulator of guanylate cyclase which causes relaxation of vascular smooth muscle and is used to treat severe pulmonary arterial hypertension. Riociguat has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury.
Background
Riociguat (rye" oh sig' ue at) is small molecular weight stimulator of soluble guanylate cyclase, an enzyme responsible for synthesis of cyclic guanine monophosphate (cyclic GMP), an important mediator of endothelial cell relaxation. By stimulating cyclic GMP, riociguat leads to relaxation of vascular smooth muscle cells, particularly in the pulmonary vasculature. In humans, riociguat induces pulmonary arterial vasodilation and reduces pulmonary artery pressure. In several clinical trials, prolonged therapy with riociguat has been shown to improve exercise capacity and pulmonary function in patients with severe idiopathic as well as chronic thromboembolic pulmonary arterial hypertension (PAH). Riociguat was approved for use in chronic idiopathic and thromboembolic PAH in 2013 and it is currently available in tablets of 0.5, 1.0, 1.5, 2.0 and 2.5 mg under the brand name Adempas. The recommended starting dose is 1 mg three times daily which can be increased in 0.5 mg amounts every two weeks based upon tolerance to a maximum of 2.5 mg thrice daily. Side effects are generally dose related and can include hypotension, syncope, dizziness, headache, diarrhea, gastrointestinal upset, nausea, vomiting and constipation, symptoms that are frequent with most vasodilator therapies. Rare, but potentially severe adverse reactions include pulmonary hemorrhage and fetal toxicity. Women of childbearing potential can receive riociguat only as a part of a risk evaluation and mitigation strategy (REMS) program that requires regular monitoring.
Hepatotoxicity
In preregistration studies, riociguat was not associated with serum enzyme elevations or with episodes of clinically apparent liver injury. Since approval of riociguat, there have been no published reports of hepatotoxicity, and the product label does not mention liver injury as an adverse event.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which riociguat might cause serum aminotransferase elevations or liver injury is not known. It is metabolized by the hepatic cytochrome P450 system (predomination 3A4 and is susceptible to drug-drug interactions. The absence of significant hepatotoxicity from riociguat may related to the relatively low daily doses used (3.0 to 7.5 mg).
Outcome and Management
The serum enzyme elevations associated with riociguat use have been rare, mild-to-moderate and self-limited in course, usually resolving despite drug continuation. Clinically apparent liver injury from riociguat has not been described. There is no information on cross sensitivity to hepatic injury among the various agents used to treat pulmonary artery hypertension.
Drug Class: Pulmonary Arterial Hypertension Agents
Other Drugs in the Subclass, Guanylate Cyclase Inhibitors: Vericiguat
Other Drugs in the Class: Ambrisentan, Bosentan, Macitentan, Selexipag; Prostacyclin Analogs, Epoprostenol, Iloprost, Treprostinil
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Riociguat – Adempas®
DRUG CLASS
Pulmonary Arterial Hypertension Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
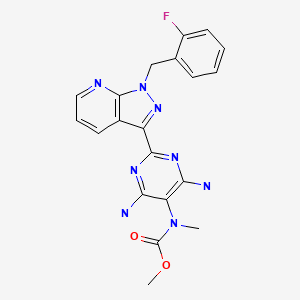
| Riociguat | 625115-55-1 | C20-H19-F-N8-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 04 June 2018
Abbreviations used: PAH, pulmonary arterial hypertension.
- Zimmerman HJ. Acetaminophen toxicity. Syndromes of environmental hepatotoxicity. In, Zimmerman, HJ. Hepatotoxicity. The adverse effects of drugs and other chemicals upon the liver. 2nd edition. Philadelphia. Lippincott, 1999. pp 337-46.(Textbook of drug induced liver injury published in 1999 before the availability of riociguat).
- Barnes PJ. Pharmacotherapy of pulmonary arterial hypertension. Pulmonary Pharmacology. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1059-61.(Textbook of pharmacology and therapeutics).
- Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40. [PubMed: 23883378](Among 443 patients with pulmonary arterial hypertension [PAH] treated with riociguat or placebo for 12 weeks, exercise capacity improved with riociguat and side effects included headache, dyspepsia, peripheral edema, nausea, dizziness and diarrhea, and one patient required early discontinuation of therapy because of serum enzyme elevations, but no details provided).
- Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29. [PubMed: 23883377](Among 261 patients with chronic thromboembolic PAH treated with riociguat or placebo for 16 weeks, exercise capacity improved with riociguat and side effects included headache, dizziness, dyspepsia, sinusitis, nausea, vomiting, diarrhea and hypotension; there were no liver related serious adverse events).
- Riociguat (Adempas) for pulmonary hypertension. Med Lett Drugs Ther. 2014;56(1437):17–9. [PubMed: 24589497](Concise review of the mechanism of action, clinical efficacy, safety and costs of riociguat shortly after its approval for use in the US mentions the common side effects and teratogenicity, but makes no mention of ALT elevations or hepatotoxicity).
- Rubin LJ, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh A, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J. 2015;45:1303–13. [PubMed: 25614164](Among 396 patients with PAH who were treated with riociguat or placebo in a randomized controlled trial and were then continued or started riociguat, improvements in exercise capacity persisted for the year of therapy and side effects included hypotension, syncope, dizziness and headache; no mention of ALT elevations or hepatotoxicity).
- Hill NS, Badesch D, Benza RL, D'Eletto TA, Farber HW, Gomberg-Maitland M, Hassoun PM, Preston I. Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Ann Am Thorac Soc. 2015;12:269–73. [PubMed: 25590376](Review of recently approved drugs for PAH including macitentan, riociguat and oral treprostinil mentions that riociguat can cause significant hypotension and is embryotoxic; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one was attributed to bosentan, but none to riociguat or other agents used primarily to treat pulmonary artery hypertension).
- Ghofrani HA, Grimminger F, Grünig E, Huang Y, Jansa P, Jing ZC, Kilpatrick D, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:361–71. [PubMed: 27067479](Among 396 patients with PAH who had participated in a placebo controlled trial and then were treated with open label riociguat, exercise capacity was maintained for the duration of therapy [up to 2 years] and no new side effects were evident; no mention of serum ALT elevations or clinically apparent liver injury).
- Simonneau G, D'Armini AM, Ghofrani HA, Grimminger F, Jansa P, Kim NH, Mayer E, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:372–80. [PubMed: 27067478](Among 237 patients with chronic thromboembolic PAH who had participated in a placebo controlled trial and then were treated with open label riociguat, exercise capacity was maintained for the duration of therapy [up to 2 years] and no new side effects were evident; no mention of serum ALT elevations or clinically apparent liver injury).
- Humbert M, Coghlan JG, Ghofrani HA, Grimminger F, He JG, Riemekasten G, Vizza CD, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76:422–6. [PMC free article: PMC5284330] [PubMed: 27457511](Analysis of 111 patients with connective tissue disease associated PAH from previous controlled trials of riociguat [Ghofrani et al 2013 and 2016] found similar rates of improvements and side effects as found in the entire treated population; no mention of ALT elevations or hepatotoxicity).
- Halank M, Hoeper MM, Ghofrani HA, Meyer FJ, Stähler G, Behr J, Ewert R, et al. Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary ypertension: Results from aphase II long-term extension study. Respir Med. 2017;128:50–6. [PubMed: 28610669](Among 68 patients with PAH treated with riociguat in a long term extension study following a controlled trial [Ghofrani 2013], clinical improvements were maintained and no new adverse events were found; no mention of ALT levels or hepatotoxicity).
- McLaughlin VV, Jansa P, Nielsen-Kudsk JE, Halank M, Simonneau G, Grünig E, Ulrich S, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med. 2017;17:216. [PMC free article: PMC5745920] [PubMed: 29282032](Among 262 patients with recurrent or persistent chronic thromboembolic PAH treated with riociguat in an open access study for an average of 1 year, common adverse events included dizziness, headache, syncope, peripheral edema and gastrointestinal symptoms; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effects of a Soluble Guanylate Cyclase Stimulator Riociguat on Contractility of Isolated Pulmonary Artery and Hemodynamics of U46619-Induced Pulmonary Hypertension in Dogs.[Vet Sci. 2023]Effects of a Soluble Guanylate Cyclase Stimulator Riociguat on Contractility of Isolated Pulmonary Artery and Hemodynamics of U46619-Induced Pulmonary Hypertension in Dogs.Kameshima S, Nakamura Y, Uehara K, Kodama T, Yamawaki H, Nishi K, Okano S, Niijima R, Kimura Y, Itoh N. Vet Sci. 2023 Feb 15; 10(2). Epub 2023 Feb 15.
- Clinical Significance of Guanylate Cyclase Stimulator, Riociguat, on Right Ventricular Functional Improvement in Patients with Pulmonary Hypertension.[Cardiology. 2021]Clinical Significance of Guanylate Cyclase Stimulator, Riociguat, on Right Ventricular Functional Improvement in Patients with Pulmonary Hypertension.Murata M, Kawakami T, Kataoka M, Moriyama H, Hiraide T, Kimura M, Endo J, Kohno T, Itabashi Y, Fukuda K. Cardiology. 2021; 146(1):130-136. Epub 2020 Nov 25.
- Review Soluble guanylate cyclase stimulators in pulmonary hypertension.[Handb Exp Pharmacol. 2013]Review Soluble guanylate cyclase stimulators in pulmonary hypertension.Stasch JP, Evgenov OV. Handb Exp Pharmacol. 2013; 218:279-313.
- The soluble guanylate cyclase stimulator riociguat and the soluble guanylate cyclase activator cinaciguat exert no direct effects on contractility and relaxation of cardiac myocytes from normal rats.[Eur J Pharmacol. 2015]The soluble guanylate cyclase stimulator riociguat and the soluble guanylate cyclase activator cinaciguat exert no direct effects on contractility and relaxation of cardiac myocytes from normal rats.Reinke Y, Gross S, Eckerle LG, Hertrich I, Busch M, Busch R, Riad A, Rauch BH, Stasch JP, Dörr M, et al. Eur J Pharmacol. 2015 Nov 15; 767:1-9. Epub 2015 Sep 25.
- Review Riociguat: a soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension.[Drug Des Devel Ther. 2017]Review Riociguat: a soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension.Lian TY, Jiang X, Jing ZC. Drug Des Devel Ther. 2017; 11:1195-1207. Epub 2017 Apr 13.
- Riociguat - LiverToxRiociguat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...