NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Riluzole is a neuroprotective agent used for therapy of amyotrophic lateral sclerosis. Riluzole is associated with a low rate of serum aminotransferase elevations during therapy and has been linked to rare instances of clinically apparent, acute liver injury.
Background
Riluzole (ril' ue zole) is a benzothiazole that is neuroprotective in vitro and in vivo. The mechanism by which it protects neurons from toxic injury is unknown, but it appears to inhibit glutamate release, to block post-synaptic glutamate receptors and to inactivate voltage dependent sodium channels. Studies in patients with amyotrophic lateral sclerosis suggest that riluzole may slow disease progression and neurologic deterioration. Riluzole was approved for use in the United States in 1995 and is commonly used in management of amyotrophic lateral sclerosis. Riluzole is available in tablets of 50 mg under the brand name Rilutek. The typical maintenance dosage is 50 mg every 12 hours. Common side effects include fatigue, weakness, dizziness, nausea, diarrhea, and cough.
Hepatotoxicity
Serum aminotransferase elevations occur in approximately up to 12% of patients on long term riluzole therapy, but elevations above 3 times the upper limit of normal (ULN) occur in less than 3% of patients. These elevations are usually mild-to-moderate in severity and are rarely associated with symptoms. Most elevations resolve spontaneously, but persistent or marked elevations require drug discontinuation or dose modification. Routine monitoring of serum aminotransferase levels is recommended for the first 6 months of therapy. Clinically apparent liver injury due to riluzole is rare, but several cases have been reported, arising after 1 to 12 months of therapy and characterized by a hepatocellular or mixed pattern of serum enzyme elevations. Immunoallergic and autoimmune features were uncommon. Most cases were mild to moderate in severity and recovery was rapid upon drug discontinuation, but evidently fatal cases have been reported to the sponsor.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
Riluzole is extensively metabolized by the liver by the cytochrome P450 system into multiple intermediates, not all of which have been characterized. The hepatotoxicity of riluzole may be related to production of a toxic or immunogenic intermediate.
Outcome and Management
The severity of liver injury from riluzole ranges from minor, transient elevations in serum aminotransferase levels to acute hepatic injury with jaundice and possible to acute liver failure. Routine monitoring of serum aminotransferase levels is recommended during the first 6 months of riluzole therapy and therapy discontinued for elevations greater than 5 times ULN. Riluzole has not been linked to cases of chronic hepatitis or vanishing bile duct syndrome. Restarting riluzole can result in recurrence of liver injury and should be avoided.
Drug Class: Alzheimer Disease Agents, Amyotrophic Lateral Sclerosis Agents
Other Related Drugs: Edaravone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Riluzole – Rilutek®
DRUG CLASS
Amyotrophic Lateral Sclerosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
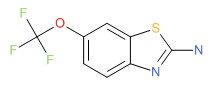
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Riluzole | 1744-22-5 | C8-H5-F3-N2-O-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 28 May 2018
- Zimmerman HJ. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 709-42.(Expert review of hepatotoxicity published in 1999; riluzole is mentioned for its potential to cause ALT elevations [~6%]).
- Larrey D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 518.(Review of hepatotoxicity of psychotropic agents published in 2007; mentions that serum ALT elevations occur in up to 12% of patients on riluzole, but that clinically apparent liver injury is rare, only 5 cases having been reported in the literature).
- Standaert DG, Roberson ED. Amyotrophic lateral sclerosis (ALT). Treatment of central nervous system degenerative disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 624-6. (Textbook of pharmacology and therapeutics)..
- Castells LI, Gamez J, Cervera C, Guardia J. Icteric toxic hepatitis associated with riluzole. Lancet 1998; 351: 648. [PubMed: 9500328](71 year old woman developed jaundice 6 months after starting riluzole for amyotrophic lateral sclerosis [bilirubin 15.4 mg/dL, ALT 1026 U/L, Alk P 356 U/L], with rapid resolution and normal values within 2 months of stopping).
- Remy A-J, Camu W, Ramos J, Blanc P, Larrey D. Acute hepatitis after riluzole administration. J Hepatol 1999; 30: 527-30. [PubMed: 10190739](Two women with amylotrophic lateral sclerosis, ages 56 and 71, developed evidence of liver injury 7 and 4 weeks after starting riluzole [bilirubin normal and 4.5 mg/dL, ALT 22 and 3 times ULN, Alk P normal], with resolution in 4-12 weeks, one patient had positive rechallenge).
- Barbare JC, Imbert A, Benkirane A. [Recent developments concerning drug- induced liver toxicity]. Presse Med 2001; 30: 673-6. French. [PubMed: 11360729](Review of importance of central reporting of drug induced liver injury, providing examples of recently described hepatotoxic reactions, including 3 recent cases of hepatocellular injury due to riluzole).
- Jankovic J, Hunter C. A double-blind, placebo-controlled and longitudinal study of riluzole in early Parkinson's disease. Parkinsonism Relat Disord 2002; 8: 271-6. [PubMed: 12039422](Among 20 patients with early Parkinson disease treated with riluzole or placebo for 12 months, none developed any abnormality in liver tests and their were no discontinuations or serious adverse events due to liver injury).
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord 2003; 4: 191-206. [PubMed: 13129806](Systematic review of safety and efficacy of riluzole identified 4 controlled trials; ALT elevations >3 times ULN were 2.6 times more frequent in riluzole than placebo treated subjects).
- Bensimon G, Doble A. The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis. Expert Opin Drug Saf 2004; 3: 525-34. [PubMed: 15500412](Review of pharmacology, efficacy and safety of riluzole in amyotrophic lateral sclerosis; ALT elevations >3 times ULN occurred in 35 [9%] of 395 patients on riluzole vs 12 of 406 [3%] on placebo; all resolved either despite continuing or with stopping: recommended monthly monitoring of aminotransferase levels for first 3 months of treatment).
- Henderson RD, McCombe PA. Riluzole: a glimmer of hope in the treatment of motor neurone disease. Med J Aust 2005; 183: 164; author reply 164-5. [PubMed: 16053424](Man in his 50s with motor neurone disease developed jaundice 11 months after starting riluzole and 3 months after starting naltrexone [bilirubin not reported, ALT 3030 U/L], resolving "gradually" after stopping both drugs).
- Wokke J. Riluzole. Lancet 1996; 348 (9030): 795-9. [PubMed: 8813989](Review of structure, mechanisms of action, pharmacokinetics, efficacy and safety of riluzole; mentions that ALT and AST elevations occur not infrequently, but are usually less than 3 times ULN and that no case of jaundice has been reported).
- Zoing MC, Burke D, Pamphlett R, Kiernan MC. Riluzole therapy for motor neurone disease: an early Australian experience (1996-2002). J Clin Neurosci 2006;13:78-83. [PubMed: 16410201](Among 25 patients with motor neurone disease treated with riluzole for up to 12 months, "there was no significant alteration" in ALT or AST).
- Landwehrmeyer GB, Dubois B, de Yébenes JG, Kremer B, Gaus W, Kraus PH, Przuntek H, et al; European Huntington's Disease Initiative Study Group. Riluzole in Huntington's disease: a 3-year, randomized controlled study. Ann Neurol 2007; 62: 262-72. [PubMed: 17702031](Among 537 adults with Huntington disease treated with riluzole or placebo for up to 3 years, 7 [2%] on riluzole and 1 [0.5%] on placebo had ALT elevations and 5 patients on riluzole stopped therapy because of liver enzyme elevations; no mention of clinically apparent liver injury or serious hepatic adverse events).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to riluzole).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to riluzole).
- Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, et al. A Prospective, Multicenter, Phase I Matched-Comparison Group Trial of Safety, Pharmacokinetics, and Preliminary Efficacy of Riluzole in Patients with Traumatic Spinal Cord Injury. J Neurotrauma 2013 Oct 11. [Epub ahead of print] [PMC free article: PMC3904533] [PubMed: 23859435](Among 36 patients with acute spinal cord injury treated with riluzole twice daily for 14 days, ALT elevaitons occurred in 70%, but elevations were usually mild-to-moderate and resolved once therapy was stopped, no patient developing jaundice or progressive increase in values).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to riluzole or other drugs for Alzheimer disease or amyotrophic lateral sclerosis).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to riluzole or drugs for Alzheimer disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 82 [9%] were attributed to central nervous system agents, but none to riluzole or drugs for Alzheimer disease).
- Mathew SJ, Gueorguieva R, Brandt C, Fava M, Sanacora G. A randomized, double-Blind, placebo-controlled, sequential parallel comparison design trial of adjunctive riluzole for treatment-resistant major depressive disorder. Neuropsychopharmacology 2017; 42: 2567-74. [PMC free article: PMC5686483] [PubMed: 28553836](Among 104 adults with major depressive disorder treated with riluzole or placebo for 4 to 8 weeks, there was no change in depression assessment scores and adverse events included headache, fatigue and body pain, and no riluzole treated subject had ALT or AST values that rose to above 5 times ULN).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Edaravone.[LiverTox: Clinical and Researc...]Review Edaravone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis.[Brain. 2013]Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis.Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, Kiernan MC. Brain. 2013 May; 136(Pt 5):1361-70.
- A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group.[N Engl J Med. 1994]A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group.Bensimon G, Lacomblez L, Meininger V. N Engl J Med. 1994 Mar 3; 330(9):585-91.
- Review The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis.[Expert Opin Drug Saf. 2004]Review The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis.Bensimon G, Doble A. Expert Opin Drug Saf. 2004 Nov; 3(6):525-34.
- Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial.[Lancet Neurol. 2018]Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial.Ludolph AC, Schuster J, Dorst J, Dupuis L, Dreyhaupt J, Weishaupt JH, Kassubek J, Weiland U, Petri S, Meyer T, et al. Lancet Neurol. 2018 Aug; 17(8):681-688. Epub 2018 Jun 19.
- Riluzole - LiverToxRiluzole - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...