NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Rifaximin is a nonabsorbable antibiotic that is used as treatment and prevention of travelers’ diarrhea and, in higher doses, for prevention of hepatic encephalopathy in patients with advanced liver disease and to treat diarrhea in patients with irritable bowel syndrome. Rifaximin has minimal oral absorption and has not been implicated in causing liver test abnormalities or clinically apparent liver injury.
Background
Rifaximin (rif ax' i min) is a synthetic antibiotic and derivative of rifamycin specifically designed to have minimal gastrointestinal absorption (<0.4%). It is a broad spectrum antibiotic with activity against both aerobic and anaerobic organisms, both gram negative and gram positive. The antibiotic activity of rifaximin is attributed to its binding to bacterial RNA polymerases, preventing RNA and subsequent protein synthesis. Rifaximin was approved for use as treatment and means of preventing travelers’ diarrhea in 2004. In 2009, the indications were expanded to include prevention of hepatic encephalopathy in patients with cirrhosis and in 2015 to treat diarrhea in patients with irritable bowel syndrome. Rifaximin is available as tablets of 200 mg under the brand name of Xifaxan for traveler’s diarrhea (recommended dose being 200 mg thrice daily for 3 days) and as tablets of 550 mg under the brand name Xifaxan 550 for hepatic encephalopathy (recommended dose being 550 mg twice daily usually in combination with lactulose) and diarrhea-predominant irritable bowel syndrome (recommended dose being 550 mg 3 times daily for 14 days). Side effects include peripheral edema, nausea, dizziness, fatigue, muscle spasms and gastrointestinal upset. Long term use has been associated with fungal or bacterial super-infections including C. difficile associated diarrhea.
Hepatotoxicity
Despite widespread use, there is little evidence that rifaximin when given orally causes liver injury, either in the form of serum enzyme elevations or clinically apparent liver disease.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Rifaximin has minimal systemic absorption and is unlikely to reach serum concentrations that might be hepatotoxic or induce CYP 3A4 enzyme activity or inhibit hepatic organic anion transport proteins, which the native compound has been shown to affect.
Other agents used for hepatic encephalopathy include neomycin and lactulose.
Drug Class: Antiinfective Agents; Gastrointestinal Agents, Antidiarrheals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Rifaximin – Xifaxan®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
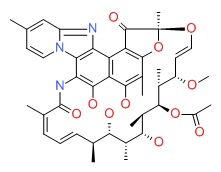
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Rifaximin | 80621-81-4 | C43-H51-N3-O11 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 14 June 2018
Abbreviations: SBP, subacute bacterial peritonitis; IBS, irritable bowel syndrome.
- Zimmerman HJ. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 598.(Expert review of hepatotoxicity published in 1999: rifamycin, but not rifaximin is discussed).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504. (Review of hepatotoxicity of antituberculosis drugs; rif.ampin can induce microsomal CYP enzymes as well as inhibit bilirubin update or excretion; rifaximin is not discussed).
- Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol 2000; 12: 203-8. [PubMed: 10741936](Controlled trial of 7 days of 3 doses of rifaximin in 54 patients with cirrhosis and hepatic encephalopathy found improvements in encephalopathy in all groups and no evidence of liver toxicity).
- DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, et al. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis 2001; 33: 1807-15. [PubMed: 11692292](187 travelers to Mexico and Jamaica with diarrhea were randomized to receive rifaximin or ciprofloxacin for 3 days; both efficacy and adverse events were similar; no mention of ALT elevations or hepatotoxicity).
- Rifaximin (Xifaxan) for travelers' diarrhea. Med Lett Drugs Ther 2004; 46: 74-5. [PubMed: 15365508](Concise review of the safety and efficacy of rifaximin for treating travelers' diarrhea shortly after its FDA approval).
- DuPont HL, Jiang ZD, Okhuysen PC, Ericsson CD, de la Cabada FJ, Ke S, DuPont MW, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea. Ann Intern Med 2005; 142: 805-12. [PubMed: 15897530](In a trial in 210 adult US student-travelers to Mexico randomized to take rifaximin or placebo for 2 weeks, there was a decrease in diarrheal illness with rifaximin [15% vs 54%]; there were no "clinically significant" patterns of laboratory abnormalities found).
- Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy 2005; 51 Suppl 1: 36-66. [PubMed: 15855748](Review of the chemical structure, absorption, antibacterial activity and clinical efficacy and safety of rifaximin; when given orally it is nontoxic in experimental animals, and few adverse events have been reported in humans, most common being flatulence, nausea, vomiting and abdominal pain with rare cases of allergic rash, but no hepatotoxicity even in post-marketing studies).
- Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med 2006; 145: 557-63. [PubMed: 17043337](87 patients with irritable bowel syndrome were treated with rifaximin or placebo for 10 days; ALT levels and hepatotoxicity were not mentioned).
- Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, Haake R, et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am J Trop Med Hyg 2006; 74: 1060-6. [PubMed: 16760520](399 US adult travelers to Mexico and India with diarrhea were treated with rifaximin, ciprofloxacin or placebo for 3 days; both agents were superior to placebo in shortening period of diarrhea with no differences in adverse events; hepatotoxicity and ALT levels were not mentioned).
- Dupont HL, Jiang ZD, Belkind-Gerson J, Okhuysen PC, Ericsson CD, Ke S, Huang DB, et al. Treatment of travelers' diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol 2007; 5: 451-6. [PubMed: 17382603](310 US students in Mexico with diarrhea were randomized to receive rifaximin, loperamide or the combination for 3 days; rifaximin was more effective than loperamide and side effects were few; no mention of hepatotoxicity or ALT elevations).
- Trehan I, Shulman RJ, Ou CN, Maleta K, Manary MJ. A randomized, double-blind, placebo-controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. Am J Gastroenterol 2009; 104: 2326-33. [PMC free article: PMC2758482] [PubMed: 19491826](144 Malawian 3 to 5 year old children were treated with rifaximin or placebo for 7 days, but no effect was found on urinary lactulose-to-mannitol ratio on a sugar absorption test [a measure of intestinal permeability]; side effects included diarrhea in 4% and rash in 4%; no mention of hepatotoxicity).
- Layer P, Andresen V. Review article: rifaximin, a minimally absorbed oral antibacterial, for the treatment of travellers' diarrhoea. Aliment Pharmacol Ther 2010; 31: 1155-64. [PubMed: 20331580](Systematic review of literature on efficacy and safety of rifaximin in travelers' diarrhea; adverse events were reported in 24-27% of patients, but were generally mild and nonspecific and similar in frequency to placebo; no mention of hepatotoxicity).
- Armstrong AW, Ulukan S, Weiner M, Mostafa M, Shaheen H, Nakhla I, Tribble DR, et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of rifaximin for the prevention of travelers' diarrhea in US military personnel deployed to Incirlik Air Base, Incirlik, Turkey. J Travel Med 2010; 17: 392-4. [PubMed: 21050319](100 US soldiers were randomized to receive rifaximin or placebo for 14 days; travelers' diarrhea occurred in 6% of rifaximin and 15% of placebo recipients; adverse events were similar in the two groups; no mention of hepatotoxicity).
- Bajaj JS, Riggio O. Drug therapy: rifaximin. Hepatology 2010; 52: 1484-8. [PubMed: 20814894](Review of the mechanism of action, efficacy and safety of rifaximin in the prevention of recurrent hepatic encephalopathy).
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362: 1071-81. [PubMed: 20335583](299 patients with cirrhosis and a history of hepatic encephalopathy were randomized to receive rifaximin or placebo for 6 months; breakthrough episodes of encephalopathy were less common on rifaximin [22%] than placebo [46%]; adverse events were similar, but C. difficile occurred in two rifaximin-, but no placebo-treated patients).
- Rifaximin (Xifaxan 550) for hepatic encephalopathy. Med Lett Drugs Ther 2010; 52: 87. [PubMed: 21045761](Concise review of rifaximin shortly after its approval for use in hepatic encephalopathy; while it reduces the rate of encephalopathy recurrence in patients with cirrhosis, it is expensive and its safety in patients with the most severe disease has not been established).
- Flores J, Dupont HL, Jiang ZD, Okhuysen PC, Melendez-Romero JH, Gonzalez-Estrada A, Carrillo I, et al. A randomized, double-blind, pilot study of rifaximin 550 mg versus placebo in the prevention of travelers' diarrhea in Mexico during the dry season. J Travel Med 2011; 18: 333-6. [PubMed: 21896097](Among 98 US travelers to Mexico treated with rifaximin [550 mg once daily] or placebo for 14 days, travelers diarrhea occurred in similar proportions of both groups [22% vs 29%]; side effects were uncommon, but one placebo recipient developed mild jaundice [bilirubin 2.7 mg/dL, AST 46 U/L], with no further details provided).
- Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, et al.; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22-32. [PubMed: 21208106](Among 1260 patients with irritable bowel syndrome [IBS] without constipation who were treated in two controlled trials of rifaximin vs placebo for 2 weeks, relief of symptoms was more frequent with rifaximin [41% vs 32%] while adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Prantera C, Lochs H, Grimaldi M, Danese S, Scribano ML, Gionchetti P; Retic Study Group (Rifaximin-Eir Treatment in Crohn's Disease). Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterology 2012; 142: 473-81. [PubMed: 22155172](Controlled trial of 3 doses of rifaximin twice daily vs placebo for 12 weeks in 402 patients with Crohn disease; one case of C. difficile, but "no clinically signficant changes in results of safety laboratory tests were observed").
- Cremonini F, Lembo A. Rifaximin for the treatment of irritable bowel syndrome. Expert Opin Pharmacother 2012; 13: 433-40. ( [PubMed: 22251066]Review of the evidence for efficacy and safety of rifaximin in IBS; "no significant adverse events have been reported during the clinical trials of rifaximin peformed to date in IBS").
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol 2013; 108: 1458-63. [PubMed: 23877348](Among 120 patients with cirrhosis treated with lactulose with or without rifaximin, hospital stays were shorter and survival was better in those receiving rifaximin; no evidence of hepatotoxicity).
- Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014; 12: 1390-7. [PubMed: 24365449](Among 392 patients with cirrhosis and hepatic encephalopathy treated with rifaximin [550 mg twice daily] for up to 24 months, adverse event rates were low and similar to historical controls; no mention of hepatotoxicity).
- Schoenfeld P, Pimentel M, Chang L, Lembo A, Chey WD, Yu J, Paterson C, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 2014; 39: 1161-8. [PMC free article: PMC4112801] [PubMed: 24697851](In a pooled analysis of 1932 patinets with IBS without constipation who were treated with rifaximin or placebo for 2 weeks, adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to rifaximin or other drugs used for hepatic encephalopathy or IBS).
- Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, Paterson C, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113-21. [PubMed: 27528177](Among 636 patients with diarrhea-predominant IBS who responded but then relapsed after therapy with rifaximin and who were then retreated with rifaximin or placebo, response rates were again higher with rifaximin while adverse event rates were similar, ALT elevations occurring in 2.1% of rifaximin vs 1.3% of placebo recipients).
- Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2016; 28: 1450-4. [PubMed: 27512927](Among 262 patients with cirrhosis, ascites and history of spontaneous bacterial peritonitis [SBP] who were given prophylaxis with rifaximin or norfloxacin, SBP rates were lower with rifaximin [4% vs 14%] as was the mortality rate [14% vs 24%], while adverse event rates were lower [15% vs 36%]).
- Suzuki K, Endo R, Takikawa Y, Moriyasu F, Aoyagi Y, Moriwaki H, Terai S, et al. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: A phase II/III, multicenter, randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatol Res 2018; 48: 411-23. (Among 172 Japanese patients with cirrhosis and hepatic encephalopathy who were treated with rifaximin or lactitol for 2 weeks, response rates were similar in the 2 groups, but adverse event rates were lower with rifaximin; no mention of ALT levels or hepatotoxicity). [PubMed: 29235218]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea.[Ann Intern Med. 2005]A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea.DuPont HL, Jiang ZD, Okhuysen PC, Ericsson CD, de la Cabada FJ, Ke S, DuPont MW, Martinez-Sandoval F. Ann Intern Med. 2005 May 17; 142(10):805-12.
- Review Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections.[Expert Rev Anti Infect Ther. 2...]Review Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections.Gerard L, Garey KW, DuPont HL. Expert Rev Anti Infect Ther. 2005 Apr; 3(2):201-11.
- Rifaximin.[StatPearls. 2024]Rifaximin.Robertson KD, Nagalli S. StatPearls. 2024 Jan
- Rifaximin for the treatment of acute infectious diarrhea.[Therap Adv Gastroenterol. 2011]Rifaximin for the treatment of acute infectious diarrhea.Hong KS, Kim JS. Therap Adv Gastroenterol. 2011 Jul; 4(4):227-35.
- Review Rifaximin: new therapeutic indication and future directions.[Clin Ther. 2011]Review Rifaximin: new therapeutic indication and future directions.Rivkin A, Gim S. Clin Ther. 2011 Jul; 33(7):812-27. Epub 2011 Jul 7.
- Rifaximin - LiverToxRifaximin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...