NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Rifamycin is a nonabsorbable rifampin-like antibacterial agent that is used as treatment of travelers’ diarrhea. Rifamycin has minimal oral absorption and has not been implicated in causing liver test abnormalities or clinically apparent liver injury.
Background
Rifamycin (rif" a mye' sin) is a semisynthetic derivative of rifampin specifically designed to have minimal gastrointestinal absorption (<0.1%). It is a broad spectrum antibiotic with activity against both aerobic and anaerobic organisms, both gram negative and gram positive. The antibiotic activity is attributed to rifamycin’s binding to bacterial RNA polymerases, preventing RNA and subsequent protein synthesis. Rifamycin is formulated with enteric coating and a multi-matrix technology that prevents the dissolving until it reaches the cecum where the higher pH (above 7) allows for the delivery of the drug throughout the colon. The delayed dissolution of the tablet prevents the antibacterial activity from acting on small bowel flora and targets it largely at the colon. This delayed release formulation of rifamycin was approved for use as treatment of travelers’ diarrhea in 2018. Rifamycin is available in tablets of 200 mg under the brand name Aemcolo. The recommended dose is 400 mg (2 tablets) twice daily for 3 days. Side effects are uncommon but can include headache and constipation. Rare instances of hypersensitivity and Clostridium difficile-associated diarrhea have been described.
Hepatotoxicity
In prelicensure controlled trials in patients with traveler’s diarrhea, rates of serum ALT elevations were similar in subjects treated with rifamycin compared to placebo or comparator agent (ciprofloxacin) and no participants developed clinically apparent liver injury. Since its approval, there have been no published reports of hepatotoxicity attributed to rifamycin. Because of its minimal absorption rifamycin is considered unlikely to cause liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Rifamycin has minimal systemic absorption and is unlikely to reach serum concentrations that might be hepatotoxic or induce CYP 3A4 enzyme activity or inhibit hepatic organic anion transport proteins, which the native compound has been shown to affect.
Other agents used for travelers’ diarrhea include rifaximin, azithromycin and ciprofloxacin.
Drug Class: Antiinfective Agents; Gastrointestinal Agents, Antidiarrheals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Rifamycin – Aemcolo®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
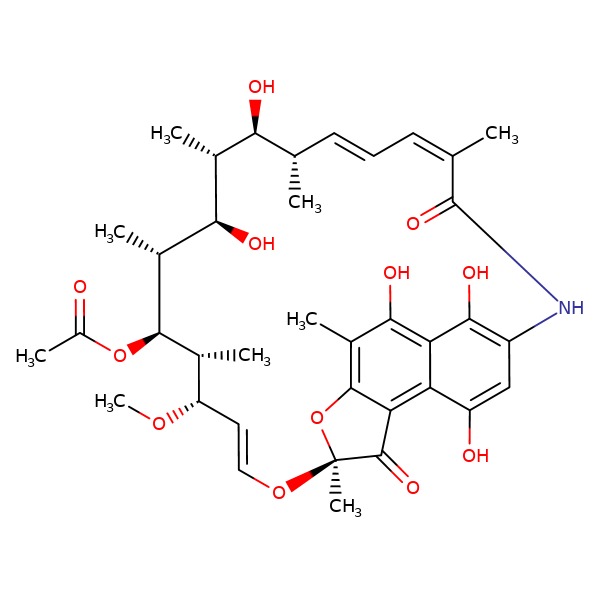
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Rifamycin | 6998-60-3 | C37-H47-N-O12 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 11 April 2019
- Zimmerman HJ. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 598.(Expert review of hepatotoxicity published in 1999; rifampin is discussed but not rifamycin or rifaximin).
- Verma S, Kaplowitz N. Hepatotoxicity of antituberculosis drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 483-504.(Review of hepatotoxicity of antituberculosis drugs; rifampin can induce microsomal CYP enzymes as well as inhibit bilirubin update or excretion; rifamycin is not discussed).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that serum enzyme elevations were uncommon during rifamycin therapy and no more frequent that with placebo or a comparator therapy [ciprofloxacin]; two subjects developed elevations in serum bilirubin with concurrent increases in ALT and AST, but in both instances the abnormalities were not thought to be due to rifamycin). - DuPont HL, Jiang ZD, Okhuysen PC, Ericsson CD, de la Cabada FJ, Ke S, DuPont MW, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea. Ann Intern Med 2005; 142: 805-12. [PubMed: 15897530](In a trial in 210 adult US student-travelers to Mexico randomized to take rifaximin or placebo for 2 weeks, there was a decrease in diarrheal illness with rifaximin [15% vs 54%]; there were no "clinically significant" patterns of laboratory abnormalities found).
- Steffen R, Hill DR, DuPont HL. Traveler's diarrhea: a clinical review. JAMA 2015; 313 (1): 71-80. [PubMed: 25562268](Review of the incidence, etiology and management of travelers’ diarrhea; rates vary from 10-40% largely dependent upon the destination and duration of exposure, as well as where meals are purchased; causes include enterotoxigenic E. coli, noroviruses, Salmonella, Shigella and Campylobacter species; prevention depends on dietary precautions; prophylaxis and treatments include bismuth subsalicylate, ciprofloxacin and rifaximin; complications may include bloody diarrhea, post-infectious irritable bowel syndrome, reactive arthritis, and Guillain-Barre syndrome).
- DuPont HL, Petersen A, Zhao J, Mundt A, Jiang ZD, Miller S, Flores J, et al. Targeting of rifamycin SV to the colon for treatment of travelers' diarrhea: a randomized, double-blind, placebo-controlled phase 3 study. J Travel Med 2014; 21: 369-76. [PubMed: 25345982](Among 264 patients with travelers’ diarrhea treated with a 3-day course of rifamycin or placebo, the duration of diarrhea was shorter [46 vs 68 hours] and clinical cure rate higher [81% vs 57%] with rifamycin, while advance event rates were lower [30% vs 38.5%] and “no drug effects on renal or liver function test results were identified”).
- Jiang ZD, DuPont HL. Etiology of travellers' diarrhea. J Travel Med 2017; 24 (suppl_1): S13-S16. [PubMed: 28521001](Review of 11 studies on the etiology of travelers’ diarrhea; overall bacterial pathogens were identified in 72-80% of cases, most commonly E. coli and for non-bacterial cases, norovirus in 3-10%).
- Steffen R, Jiang ZD, Gracias Garcia ML, Araujo P, Stiess M, Nacak T, GreinwaldR, DuPont HL. Rifamycin SV-MMX® for treatment of travelers' diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria. J Travel Med 2018; 25 (1). [PMC free article: PMC6331114] [PubMed: 30462260](Among 835 travelers with diarrhea treated with rifamycin [400 mg twice daily] or ciprofloxacin [500 mg twice daily] for 3 days, time to cure was similar [43 vs 37 hours] as were adverse event rates [8.1% vs 7.5%] and no serious adverse events occurred).
- Rifamycin (Aemcolo) for treatment of travelers' diarrhea. Med Lett Drugs Ther 2019; 61 (1567): 39-40. [PubMed: 30845098](Concise review of the mechanism of action, clinical efficacy, safety and costs of rifamycin shortly after its approval as therapy of travelers' diarrhea in the US, does not mention ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Rifaximin.[LiverTox: Clinical and Researc...]Review Rifaximin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea.[Ann Intern Med. 2005]A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea.DuPont HL, Jiang ZD, Okhuysen PC, Ericsson CD, de la Cabada FJ, Ke S, DuPont MW, Martinez-Sandoval F. Ann Intern Med. 2005 May 17; 142(10):805-12.
- Targeting of rifamycin SV to the colon for treatment of travelers' diarrhea: a randomized, double-blind, placebo-controlled phase 3 study.[J Travel Med. 2014]Targeting of rifamycin SV to the colon for treatment of travelers' diarrhea: a randomized, double-blind, placebo-controlled phase 3 study.DuPont HL, Petersen A, Zhao J, Mundt A, Jiang ZD, Miller S, Flores J, Shringarpure R, Moro L, Bagin RG, et al. J Travel Med. 2014 Nov-Dec; 21(6):369-76.
- Review Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections.[Expert Rev Anti Infect Ther. 2...]Review Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections.Gerard L, Garey KW, DuPont HL. Expert Rev Anti Infect Ther. 2005 Apr; 3(2):201-11.
- Rifamycin (Aemcolo) for treatment of travelers' diarrhea.[Med Lett Drugs Ther. 2019]Rifamycin (Aemcolo) for treatment of travelers' diarrhea.. Med Lett Drugs Ther. 2019 Mar 11; 61(1567):39-40.
- Rifamycin - LiverToxRifamycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...