NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Protriptyline is a tricyclic antidepressant that was previously widely used in the therapy of major depression. Most of the tricyclic antidepressants have been shown to cause a low rate of mild and transient serum enzyme elevations and rare cases of clinically apparent acute cholestatic liver injury. The potential hepatotoxicity specifically of protriptyline, however, has not been well defined.
Background
Protriptyline (proe trip' ti leen) is a tricyclic antidepressant which acts by inhibition of serotonin and norepinephrine reuptake within synaptic clefts in the central nervous system, thus increasing brain levels of these neurotransmitters. Protriptyline is indicated for therapy of major depression and was approved for this indication in the United States in 1967, but is no longer widely used, having been replaced by the selective serotonin reuptake inhibitors (SSRIs) and other better tolerated and more easily administered agents. Protriptyline, unlike other tricyclic antidepressants, tends to be energizing rather than sedating and is used off label for narcolepsy, sleep apnea and attention deficit disorder. Protriptyline is available in generic forms and under the brand name of Vivactil in 5 and 10 mg tablets. The typical recommended dose for depression in adults is 15 to 40 mg daily in 3 to 4 divided doses. Common side effects include dizziness, headache, drowsiness, restlessness, confusion, tachycardia, gastrointestinal upset, increased appetite, weight gain, blurred vision, dry mouth and urinary retention.
Hepatotoxicity
Liver test abnormalities have been reported to occur in 10% to 12% of patients on tricyclic antidepressants, but elevations are uncommonly above 3 times the upper limit of normal. The aminotransferase abnormalities are usually mild, asymptomatic and transient, reversing even with continuation of medication. The rate of serum enzyme elevations specifically during protriptyline therapy has not been well defined. Rare instances of clinically apparent acute liver injury have been reported in patients on tricyclic antidepressants, but there have been no specific reports related to protriptyline. In typical tricyclic antidepressant acute liver injury, the latency to onset has ranged from 1 to 14 months. The pattern of serum enzyme elevations was typically cholestatic, but hepatocellular cases have also been reported including an acute hepatitis-like syndrome with acute liver failure. Instances of acute cholestatic hepatitis and prolonged jaundice compatible with vanishing bile duct syndrome have been linked to other tricyclic antidepressants, mostly amitriptyline and imipramine, the two most commonly used agents in this class. Signs or symptoms of hypersensitivity (rash, fever and eosinophilia) are frequent in reported cases, but these symptoms are usually mild and transient. Autoantibody formation is rare. Protriptyline is a rarely used tricyclic antidepressant but is suspected of having a profile of adverse effects similar to that of imipramine and amitriptyline.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which protriptyline might cause liver injury is not known. It undergoes extensive hepatic metabolism and a possible cause of liver injury is production of a toxic intermediate of metabolism. Many cases have features of hypersensitivity, and more rapid recurrence with reexposure and some instances of tricyclic antidepressant liver injury have been associated with a specific HLA haplotype (A11).
Outcome and Management
The serum aminotransferase elevations that occur on protriptyline therapy are usually self-limited and do not require dose modification or discontinuation of therapy. The acute liver injury caused by tricyclic antidepressants is typically self-limited, but progressive and fatal instances of acute hepatitis and prolonged cholestasis with vanishing bile duct syndrome have been reported. Rechallenge with the same tricyclic antidepressant usually causes a prompt recurrence of the liver injury which can be fatal and should be avoided. Cross reactivity of hepatic injury with other tricyclic antidepressants has been described but is not invariable. Thus, switching from one to another tricyclic antidepressant after clinically apparent liver injury should be avoided or done with caution. Switching to other forms of antidepressants such as the selective serotonin reuptake inhibitors is likely to be safe.
Drug Class: Antidepressant Agents
Other Drugs in the Subclass, Tricyclics: Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Nortriptyline, Trimipramine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Protriptyline – Generic, Vivactil®
DRUG CLASS
Antidepressant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
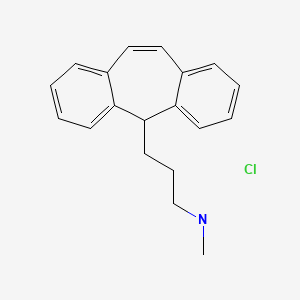
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Protriptyline Hydrochloride | 438-60-8 | C19-H21-N |
|
ANNOTATED BIBLIOGRAPHY
References updated: 05 April 2020
Abbreviations: MAO inhibitor, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor.
- Zimmerman HJ. Tricyclic antidepressants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp.495-8.(Expert review of hepatotoxicity published in 1999; hepatic injury caused by tricyclic antidepressants is less frequent and less consistent than with monoamine oxidase inhibitors).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of tricyclic antidepressant hepatotoxicity; protriptyline is not specifically discussed but there is some cross-reactivity to hepatic injury among the tricyclic antidepressants).
- O'Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-77.(Textbook of pharmacology and therapeutics).
- Klerman GL, Cole JO. Clinical pharmacology of imipramine and related antidepressant compounds. Pharmacol Rev. 1965;17:101–41. [PubMed: 14294030](Extensive review of structure, pharmacology, clinical effects, mechanisms of action, drug interactions, and side effects of tricyclic antidepressants; jaundice occurs in 0.5-1% of treated persons and usually resolves rapidly with stopping).
- Bercel NA. Clinical trial of protriptyline (Vivactil). Int J Neuropsychiatry. 1967;3(4):365–78. [PubMed: 4863509](Analysis of industry sponsored studies of protriptyline in 150 patients with psychiatric disorders; AST and Alk P levels were tested in 79 patients, none of which were elevated or changed from before therapy).
- Protriptyline HCl. (Vivactil HCl). Clin Pharmacol Ther. 1968;9:409–12. [PubMed: 5754330](Summary of the uses and side effects of protriptyline from the sponsor; no mention of ALT elevations or liver injury).
- Protriptyline (Vivactil): another antidepressant. Med Lett Drugs Ther. 1968;10:17. [PubMed: 5645606](Concise review of the efficacy and safety of protriptyline shortly after its approval in the US; common side effects are dry mouth, blurred vision, urinary retention, and tachycardia; mentions that "cholestatic jaundice is a possible complication, as with other tricyclics").
- Isaksson A, Larkander O, Morsing C, Ottosson JO, Rapp W. A controlled comparison between imipramine and protriptyline. Acta Psychiatr Scand Suppl. 1968;203:239–41. [PubMed: 4881878](Controlled trial of imipramine vs protriptyline in 76 adults with depression found no change in ALT levels during therapy with either agent).
- Greenblatt DJ, Koch-Weser J, Shader RI. Multiple complications and death following protriptyline overdose. JAMA. 1974;229:556–7. [PubMed: 4209436](28 year old man took overdose [unknown quantity] of protriptyline and presented with hypotension, seizures and respiratory failure progressing to coma, acidosis and renal failure; autopsy showed hepatic congestion and fatty change).
- Hollister LE. Tricyclic antidepressants (first of two parts). N Engl J Med. 1978;299:1106–9. [PubMed: 30045]
- Hollister LE. Tricyclic antidepressants (second of two parts). N Engl J Med. 1978;299:1168–72. [PubMed: 703806](Review of mechanism of action, efficacy and safety of the tricyclic antidepressants including protriptyline; serious side effects include confusional reactions, weight gain, and cardiovascular events; no mention of hepatotoxicity or ALT elevations).
- Brownell LG, West P, Sweatman P, Acres JC, Kryger MH. Protriptyline in obstructive sleep apnea: a double-blind trial. N Engl J Med. 1982;307:1037–42. [PubMed: 6750396](Crossover controlled trial of 2 weeks of protriptyline vs placebo followed by long term open label use in 5 men with sleep apnea, found no untoward drug events except urinary hesitancy).
- Cassidy S, Henry J. Fatal toxicity of antidepressant drugs in overdose. Br Med J(Clin Res Ed). 1987;295(6605):1021–4. [PMC free article: PMC1248068] [PubMed: 3690249](Analysis of National Health Service records of prescriptions of antidepressant drugs and deaths from suicides between 1975 and 1984 in the UK found deaths per million prescriptions highest with desipramine [80] and amitriptyline [47], somewhat lower with doxepin [31] and imipramine [28], and lowest with protriptyline [10]).
- Fiori MG. Tricyclic antidepressants: a review of their toxicology. Curr Dev Psychopharmacol. 1977;4:71–110. [PubMed: 340145](Review of cardiac, hepatic, neurological, fetal and psychotoxicity of tricyclic antidepressants; most cases of hepatotoxicity have been attributed to hypersensitivity, but tricyclics are taken up and extensively metabolized by hepatocytes).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Among 572 cases of drug induced liver disease seen between 1968-78 in Denmark, psychotropic agents accounted for 93 cases, 54 of which were due to chlorpromazine; tricyclics not specifically mentioned).
- Larrey D, Rueff B, Pessayre D, Algard M, Geneve J, Benhamou JP. Cross hepatotoxicity between tricyclic antidepressants. Gut. 1986;27:726–7. [PMC free article: PMC1433318] [PubMed: 3721296](39 year old woman developed abdominal pain 2 weeks after starting amineptine [a tricyclic antidepressant] with fever and eosinophilia [bilirubin 1.2 mg/dL, ALT 1360 U/L, Alk P 1.5 times ULN], resolving rapidly upon stopping and recurring 7 days after starting clomipramine [another tricyclic] [ALT 1050 U/L, Alk P 1.5 times ULN], again resolving rapidly upon stopping).
- Genève J, Larrey D, Pessayre D, Benhamou JP. Structure tricyclique des medicaments et hepatotoxicite. Gastroenterol Clin Biol. 1987;11:242–9. [PubMed: 2884161](Review of structural similarity and hepatotoxicity of tricyclic antidepressants focusing on amineptine, imipramine and amitriptyline).
- Larrey D, Amouyal G, Pessayre D, Degott C, Danne O, Machayekhi JP, Feldmann G, et al. Amitriptyline-induced prolonged cholestasis. Gastroenterology. 1988;94:200–3. [PubMed: 3335290](37 year old man developed jaundice 5 weeks after starting amitriptyline [bilirubin 5.9 mg/dL, ALT 6.5 times ULN, Alk P 1.3 times ULN]; the drug was continued and bilirubin peaked at 23.4 mg/dL with 8% eosinophils and subsequent prolonged jaundice and pruritus [19-20 months] and ductopenia on liver biopsy).
- Pirmohamed M, Kitteringham NR, Park BK. Idiosyncratic reactions to antidepressants: a review of the possible mechanisms and predisposing factors. Pharmacol Ther. 1992;53:105–25. [PubMed: 1641399](Review of idiosyncratic reactions to antidepressants; possible mechanism of injury being production of a chemically reactive metabolite that is either directly toxic or induces a hypersensitivity reaction).
- Berson A, Fréneaux E, Larrey D, Lepage V, Douay C, Mallet C. Possible role of HLA in hepatotoxicity. An exploratory study. J Hepatol. 1994;20:336–42. [PubMed: 8014443](Human leukocyte antigen [HLA] haplotypes done on 71 patients with drug induced liver disease; 12 due to tricyclics including 7 amineptine, 3 amitriptyline and 2 clomipramine; 6 [50%] had HLA A11 including 2 of the 3 amitriptyline cases; 12% in controls).
- Remy AJ, Larrey D, Pageaux GP, Ribstein J, Ramos J, Michel H. Cross hepatotoxicity between tricyclic antidepressants and phenothiazines. Eur J Gastroenterol Hepatol. 1995;7:373–6. [PubMed: 7600146](65 year old woman developed fatigue and serum enzyme elevations [ALT ~1300 U/L; Alk P ~380 U/L] 1 month after starting trimipramine; 3 years later she developed nausea and ALT elevations 10 days after starting desipramine [ALT ~250 U/L], and 2 years later developed abdominal pain and fever and enzyme elevations [ALT ~1100 U/L, Alk P ~510 U/L] 8 days after starting cyamemazine; each time with rapid recovery and no jaundice).
- Grohmann R, Rüther E, Engel RR, Hippius H. Assessment of adverse drug reactions in psychiatric inpatients with the AMSP drug safety program: methods and first results for tricyclic antidepressants and SSRIs. Pharmacopsychiatry. 1999;32:21–8. [PubMed: 10071179](Analysis of reporting of adverse events among inpatients in 29 German hospitals between 1993 to 1997; 896 severe adverse events among 48,564 patients [1.8%], both total and hepatic events were more common with tricyclics than SSRIs).
- Carvajal García-Pando A, García del Pozo J, Sánchez AS, Velasco MA, Rueda de Castro AM, Lucena MI. Hepatotoxicity associated with the new antidepressants. J Clin Psychiatry. 2002;63:135–7. [PubMed: 11874214](Analysis of cases of hepatotoxicity from antidepressants in Spanish Pharmacovigilance System from 1989-1999, identified 99 cases including 31 due to tricyclics: 16 clomipramine 7 amitriptyline, 6 imipramine [protriptyline not mentioned]).
- Lucena MI, Carvajal A, Andrade RJ, Velasco A. Antidepressant-induced hepatotoxicity. Expert Opin Drug Saf. 2003;2:249–62. [PubMed: 12904104](Review of hepatotoxicity of antidepressants; antidepressant use has increased markedly between 1992 and 2002, accounting for 5% of cases of hepatotoxicity; tricyclics less likely to cause injury than MAO inhibitors; predominantly cholestatic patterns with onset in first 2-3 weeks; occasional reports of prolonged cholestasis).
- Degner D, Grohmann R, Kropp S, Rüther E, Bender S, Engel RR, Schmidt LG. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37 Suppl 1:S39–45. [PubMed: 15052513](53,042 patients treated with antidepressants in 35 psychiatric hospitals in Germany from 1993-2000 were monitored for adverse drug reactions; increased liver enzymes reported in 16% on tricyclics, 5.5% on SSRIs and 12% of monoamine oxidase inhibitors).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 3 were due to amitriptyline with a relative risk of 14.2: estimated frequency of 6 per 100,000 person-year exposures).
- DeSanty KP, Amabile CM. Antidepressant-induced liver injury. Ann Pharmacother. 2007;41:1201–11. [PubMed: 17609231](Review of drug induced liver injury and summary analysis of reports of injury from MAO inhibitors, SSRIs, tricyclics and atypical agents).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network(DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, only 1 case was attributed to amitriptyline, no other tricyclic mentioned).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury but none were linked to tricyclic antidepressants).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N. Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, half [n=15] were due to antimicrobials [minocycline 4, INH 3, azithromycin 3] and the rest largely due to anticonvulsants and CNS agents; one case was attributed to amitriptyline, but no other tricyclic antidepressant was implicated).
- Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8:207–23. [PubMed: 23914755](Review of drug induced liver injury due to antidepressants; protriptyline is not mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to tricyclic antidepressants).
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–15. [PubMed: 24362450](Review of the frequency and clinical features of drug induced liver injury due to antidepressants; imipramine, desipramine, amitriptyline and clomipramine are discussed, but not nortriptyline).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to an antidepressant [amitriptyline] and none to a MAO inhibitor, SSRI or SNRI).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases [2%] were attributed to antidepressants including 9 due to SNRIs [7 to duloxetine, 1 each to nefazodone and trazodone], 5 to bupropion, 5 to SSRIs [3 to escitalopram, and 1 each to fluoxetine and sertraline], and only 1 to tricyclics [imipramine], but none to protriptyline).
- Woo HJ, Kim HY, Choi ES, Cho YH, Kim Y, Lee JH, Jang E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine. 2015;22:1201–5. [PubMed: 26598920](Among 1169 inpatients seen at a single Korean referral medical center over a 2 year period, 11 developed suspected drug induced liver injury, 6 attributed to dietary supplements and 5 to conventional drugs including 2 antidepressants [minocycline, donepezil, warfarin, gabapentin/milnacipran, and antihistamines]).
- Voican CS, Martin S, Verstuyft C, Corruble E, Perlemuter G, Colle R. Liver function test abnormalities in depressed patients treated with antidepressants: a real-world systematic observational study in psychiatric settings. PLoS One. 2016;11:e0155234. [PMC free article: PMC4865191] [PubMed: 27171561](Among 321 psychiatric inpatients, only 116 [36%] had liver tests performed and only 18 during therapy with an antidepressant, 3 of which were suspected to have drug induced liver injury, 1 each with escitalopram, venlafaxine and amitriptyline, all without jaundice and 2 without symptoms, all 3 resolving).
- Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2016;19(4):pyv126. pii. [PMC free article: PMC4851269] [PubMed: 26721950](Among 184,234 psychiatric inpatients from 80 hospitals, 149 cases [0.08%] of drug induced liver injury were reported including 71 of 50,201 patients treated with tricyclics, 18 of 11,876 receiving trimipramine and 2 of 2,016 receiving nortriptyline; protriptyline not mentioned).
- Gahr M, Zeiss R, Lang D, Connemann BJ, Hiemke C, Schönfeldt-Lecuona C. Drug-Induced liver injury associated with antidepressive psychopharmacotherapy: an explorative assessment based on quantitative signal detection using different MedDRA terms. J Clin Pharmacol. 2016;56:769–78. [PubMed: 26470856](Using data on adverse drug reaction reports from the Uppsala Monitoring Center of WHO, there were higher relative hepatotoxicity reports for nefazodone, agomelatine, many tricyclics and mirtazapine).
- Chen VC, Lin CF, Hsieh YH, Liang HY, Huang KY, Chiu WC, Lee Y, McIntyre RS, et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2017;8:30464–70. [PMC free article: PMC5444756] [PubMed: 27783998](Among almost 50,000 cases of hepatocellular carcinoma registered in the Taiwan National Health Insurance Research Database, the rate of antidepressant use was lower than in approximately 250,000 matched controls from the database).
- Ferrajolo C, Scavone C, Donati M, Bortolami O, Stoppa G, Motola D, Vannacci A, et al. DILI-IT Study Group. Antidepressant-Induced Acute liver injury: a case-control study in an Italian inpatient population. Drug Saf. 2018;41:95–102. [PubMed: 28770534](Among 179 cases of hospitalizations for unexplained acute liver injury enrolled in an Italian prospective study between 2010 and 2014, 17 had been exposed to antidepressants including citalopram [n=4], sertraline [n=3], amitriptyline [n=3] and paroxetine [n=2], clomipramine [n=1] and amitriptyline [n=1]).
- Billioti de Gage S, Collin C, Le-Tri T, Pariente A, Bégaud B, Verdoux H, Dray-Spira R, et al. Antidepressants and hepatotoxicity: a cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs. 2018;32:673–84. [PMC free article: PMC6061298] [PubMed: 29959758](Among 5 million persons identified in a national French health insurance database who started an antidepressant between 2010 and 2015, 382 developed serious liver injury resulting in hospitalization, rates per 100,0000 persons-years being 19 for SSRIs, 22 venlafaxine, 13 duloxetine, and 33 mirtazapine; conventional tricyclics and MAO inhibitors not discussed).
- Pladevall-Vila M, Pottegård A, Schink T, Reutfors J, Morros R, Poblador-Plou B, Timmer A, et al. Risk of acute liver injury in agomelatine and other antidepressant users in four European countries: a cohort and nested case-control study using automated health data sources. CNS Drugs. 2019;33:383–95. [PMC free article: PMC6441103] [PubMed: 30830574](Analysis of data sources from 4 European countries identified 3.2 million persons initiating antidepressant therapy among whom there was no increased risk for acute liver injury for agomelatine compared to citalopram, an SSRI with a low rate of hepatotoxicity).
- Drugs for depression. Med Lett Drugs Ther. 2020;62(1592):25–32. [PubMed: 32320387](Concise review of the mechanism of action, clinical efficacy, safety and costs of drugs for depression, mentions that tricyclics and MAO inhibitors remain valuable alternatives for treatment of moderate-to-severe depression, despite concerns about their safety; hepatotoxicity is mentioned only for nefazodone [now rarely used because of severe hepatotoxicity] and duloxetine [in heavy drinkers]).
- Ueberberg B, Frommberger U, Messer T, Zwanzger P, Kuhn J, Anghelescu I, Ackermann K, et al. Drug-induced liver injury (DILI) in patients with depression treated with antidepressants: a retrospective multicenter study. Pharmacopsychiatry. 2020;53:60–4. [PubMed: 31958850](Among 329 psychiatric inpatients with depression seen at 6 psychiatric centers in Germany, 17 [5%] had serum aminotransferase elevations but none had clinically apparent liver injury, most commonly implicated drugs included mirtazapine, agomelatine, citalopram and venlafaxine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Antidepressant Agents.[LiverTox: Clinical and Researc...]Review Antidepressant Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Amitriptyline.[LiverTox: Clinical and Researc...]Review Amitriptyline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Doxepin.[LiverTox: Clinical and Researc...]Review Doxepin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Desipramine.[LiverTox: Clinical and Researc...]Review Desipramine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Imipramine.[LiverTox: Clinical and Researc...]Review Imipramine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Protriptyline - LiverToxProtriptyline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...