NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Plerixafor is a small molecular antagonist of the cell-surface CXCR4 receptor that plays an important role in mobilization of hematopoietic stem and progenitor cells to the stroma of the bone marrow; blocking the receptor helps to mobilize stem cells from the marrow to peripheral blood allowing for collection of these cells by apheresis for hematopoietic cell transplantation. Plerixafor is generally well tolerated and has not been linked to serum enzyme elevations or to clinically apparent liver injury.
Background
Plerixafor (pler ix' a for) is a small molecule antagonist of CXCR4 that blocks its down-steam signaling and results in release of CD34+ hematopoietic stem and progenitor cells from the bone marrow, enabling their collection by apheresis to be used in hematopoietic cell transplantation. In multiple clinical studies, the combination of plerixafor with granulocyte-colony stimulating factor (G-CSF) resulted in greater mobilization in CD34+ cells than G-CSF alone and more successful retrieval of hematopoietic stem cells from donors and better engraftment in recipients. Plerixafor, in combination with G-CSF, was approved for use in mobilizing hematopoietic stem cells for autologous transplantation in patients with non-Hodgkin lymphoma and multiple myeloma in the United States in 2008 and it has become commonly used for both allogeneic and autologous hematopoietic cell transplantation. Plerixafor is available in solution in 1.2 mL single-use vials of 20 mg/mL. The recommended dose is 0.24 mg/kg after the patient or potential donor has received G-CSF once daily for 4 days. The dose of plerixafor can be repeated for up to 4 days. Common adverse reactions include diarrhea, nausea, fatigue, injection site reactions, headache, dizziness and arthralgia. Rare, but potential severe adverse reactions include tumor cell mobilization in leukemia patients, splenic rupture and fetal-embryonal toxicity.
Hepatotoxicity
Plerixafor has not been linked to instances of significant serum enzyme elevations during therapy nor to cases of clinically apparent liver injury. In multiple large prelicensure as well as postmarketing controlled trials, neither ALT elevations or acute liver injury were mentioned as adverse events or reasons for drop out, early discontinuation of therapy or dose modification. There have been no published reports of liver injury attributed to plerixafor, and it has been used as a possible means of treatment in animal models of acute liver failure. Thus, clinically apparent liver injury due to plerixafor must be rare, if it exists at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which plerixafor might cause liver injury is not known. It is given in low total dosage for 1 to 4 days only.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal arising de novo during hematopoietic cell mobilization should lead to discontinuation. Plerixafor has not been implicated in causing clinically apparent hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no reason to suspect any degree of cross sensitivity in risk for hepatic injury among the various hematologic growth factors and other agents used to mobilize blood marrow cells.
Drug Class: Hematologic Agents, Transplant Agents
Other Drugs in the Class, Transplant Agents: Cyclosporine, Everolimus, Mycophenolate, Sirolimus
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Plerixafor – Mozobil®
DRUG CLASS
Transplant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
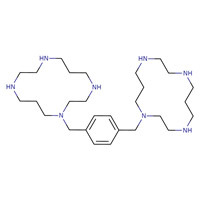
| Plerixafor | 110078-46-1 | C28-H54-N8 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 12 April 2019
Abbreviations: G-CSF, granulocyte colony stimulating factor; HCT, hematopoietic cell transplantation.
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999; plerixafor is not mentioned).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; plerixafor is not discussed).
- Kaushansky K, Kipps TJ. Hematopoietic agents: growth factors, minerals, and vitamins. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 751-68.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; does not mention or discuss serum enzyme elevations or hepatotoxicity). - Stahel RA, Jost LM, Cerny T, Pichert G, Honegger H, Tobler A, Jacky E, Fey M, Platzer E. Randomized study of recombinant human granulocyte colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation for high-risk lymphoid malignancies. J Clin Oncol 1994; 12: 1931-8. [PubMed: 7521907](Among 43 patients with lymphomas undergoing chemotherapy and hematopoietic cell transplant ation [HCT] given filgrastim in two different doses, efficacy and tolerance were similar and the only adverse events attributed to the G-CSF were bone pain and skin reactions).
- DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, et al.; 3102 Investigators. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720-6. [PubMed: 19363221](Among 308 patients with multiple myeloma who were eligible for autologous HCT and received G-CSF for 4 days followed by G-CSF with plerixafor or placebo for 1-4 days, successful mobilization of CD34+ cells was more frequent and more rapid with plerixafor, but 1 year survival was the same as were rates of adverse events, no mention of ALT elevations or hepatotoxicity).
- DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P. Plerixafor. Nat Rev Drug Discov 2009; 8: 105-6. [PubMed: 19180104](Short review of the mechanism of action of plerixafor and its use in mobilizing hematopoietic stem cells for transplantation).
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, et al.; 3101 Investigators. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009; 27: 4767-73. [PubMed: 19720922](Among 298 patients with non-Hodgkin lymphoma eligible for HCT who underwent stem cell mobilization with G-CSF and either plerixafor or placebo, successful and rapid mobilization was more frequent with plerixafor [59% vs 20%], while common adverse events included diarrhea [38% vs 6%], nausea [17% vs 6%], abdominal pain [6% vs 1%], injection site reactions, bone pain [11% vs 7%] and headache [11% vs 6%], no mention of ALT elevations or hepatotoxicity).
- Plerixafor (Mozobil). Med Lett Drugs Ther 2010; 52 (1335): 27-8. [PubMed: 20360661](Concise review of the mechanism of action, clinical efficacy, safety and costs of plerixafor shortly after its approval for use in the US; does not mention ALT elevations or hepatotoxicity).
- Brave M, Farrell A, Ching Lin S, Ocheltree T, Pope Miksinski S, Lee SL, Saber H, et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 2010; 78: 282-8. [PubMed: 20530974](An FDA review of the clinical efficacy and safety of plerixafor from two large, registration trials that led to its approval for mobilization of hematopoietic stem cells for use in autologous HCT; no mention of ALT elevations or hepatotoxicity).
- Lemery SJ, Hsieh MM, Smith A, Rao S, Khuu HM, Theresa D, Viano JM, et al. A pilot study evaluating the safety and CD34+ cell mobilizing activity of escalating doses of plerixafor in healthy volunteers. Br J Haematol 2011; 153: 66-75. [PMC free article: PMC3879799] [PubMed: 21352197](Among 21 healthy volunteers who received 1 to 2 single doses of plerixafor, common adverse events included diarrhea, bloating, nausea, injection site reactions, headache and peroral numbness).
- Russell N, Douglas K, Ho AD, Mohty M, Carlson K, Ossenkoppele GJ, Milone G, et al. Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: results of the prospective PREDICT trial. Haematologica 2013; 98: 172-8. [PMC free article: PMC3561422] [PubMed: 22983579](Among 118 patients with non-Hodgkin lymphoma or multiple myeloma undergoing stem cell mobilization in preparation for autologous HCT who received G-CSF and plerixafor, treatment emergent events included diarrhea, nausea, injection-site reactions and bone pain; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury seen over a ten-year period at 8 US medical centers, none were attributed to the agents used for hematopoietic stem cell mobilization).
- Pantin J, Purev E, Tian X, Cook L, Donohue-Jerussi T, Cho E, Reger R, et al. Effect of high-dose plerixafor on CD34(+) cell mobilization in healthy stem cell donors: results of a randomized crossover trial. Haematologica 2017; 102: 600-9. [PMC free article: PMC5394957] [PubMed: 27846612](Among 23 healthy subjects receiving plerixafor [240 or 480 µg/kg], the higher dose resulted in higher peak numbers of CD34+ cells in peripheral blood and adverse event rates were similar, only one subject [after the lower dose] had a mild and transient ALT elevation).
- Ahmadi AR, Chicco M, Wesson RN, Anders RA, Dor FJMF, Ijzermans JNM, Creamer TJ, et al. Stem cell mobilization is lifesaving in a large animal preclinical model of acute liver failure. Ann Surg 2018; 268: 620-31. [PubMed: 30102635](In a pig model of acute liver failure induced by d-galactosamine, administration of G-CSF and plerixafor at 0, 1 and 2 days was associated with higher survival and less severe hepatic injury).
- Chen YB, Le-Rademacher J, Brazauskas R, Kiefer DM, Hamadani M, DiPersio JF, Litzow MR, et al. Plerixafor alone for the mobilization and transplantation of HLA-matched sibling donor hematopoietic stem cells. Blood Adv 2019; 3: 875-83. [PMC free article: PMC6436017] [PubMed: 30890544](Among 64 healthy donors receiving a single dose of plerixafor alone [240 µg/kg] followed by leukapheresis 4 hours later, adequate mobilization of CD4+ cells was achieved in 98% and adverse events were generally mild; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.[Clin Ther. 2010]Review Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Steinberg M, Silva M. Clin Ther. 2010 May; 32(5):821-43.
- Review Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma.[Drugs. 2011]Review Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma.Keating GM. Drugs. 2011 Aug 20; 71(12):1623-47.
- Review Plerixafor: a peripheral blood stem cell mobilizer.[Pharmacotherapy. 2010]Review Plerixafor: a peripheral blood stem cell mobilizer.Kessans MR, Gatesman ML, Kockler DR. Pharmacotherapy. 2010 May; 30(5):485-92.
- Review Optimizing mobilization strategies in difficult-to-mobilize patients: The role of plerixafor.[Transfus Apher Sci. 2015]Review Optimizing mobilization strategies in difficult-to-mobilize patients: The role of plerixafor.Goker H, Etgul S, Buyukasik Y. Transfus Apher Sci. 2015 Aug; 53(1):23-9. Epub 2015 Jun 9.
- A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma.[Biol Blood Marrow Transplant. ...]A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma.Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, DiPersio J. Biol Blood Marrow Transplant. 2008 Nov; 14(11):1253-61.
- Plerixafor - LiverToxPlerixafor - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...