NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mycophenolate mofetil is an antimetabolite and potent immunosuppressive agent used as adjunctive therapy in prevention of allograft rejection and in the treatment of serious autoimmune diseases. Mycophenolate therapy can be associated with mild serum enzyme elevations and it has been linked to rare instances of clinically apparent liver injury.
Background
Mycophenolate (mye" koe fen' o late) mofetil (moe' fe til) is an antimetabolite and immunosuppressive agent that is used widely in the prevention of rejection after organ transplantation as well as in management of patients with serious autoimmune diseases. Mycophenolate blocks de novo purine synthesis via noncompetitive inhibition of inosine monophosphate dehydrogenase (IMPDH). Lymphocytes lack the salvage pathway for purine synthesis and are particularly vulnerable to the inhibitory activity of mycophenolate. The result of the IMPDH inhibition is an inhibition of lymphocyte proliferation and function. Mycophenolate mofetil was approved for use in the United States in 1995 and its current indications are for prevention of organ rejection after transplantation. It is also used off label in therapy of active and recalcitrant forms of autoimmune diseases. Mycophenolate is available as capsules of 250 mg and tablets of 500 mg in several generic forms and under the brand name CellCept, and as an oral suspension (200 mg/mL) and a powder for injection (500 mg). It is also available as mycophenolic acid in 180 and 360 mg delayed release tablets under the brand name Myfortic. The typical maintenance dose is 1 to 1.5 grams twice daily. Common side effects include gastrointestinal upset, diarrhea, nausea, headache, fatigue and dizziness. Uncommon but potentially severe adverse events include blood dyscrasias, gastrointestinal bleeding or ulceration, increased risk for lymphoma or other malignancies, increased risk for serious infections, and embryo-fetal toxicity.
Hepatotoxicity
Serum enzyme elevations occur in a small proportion of patients on mycophenolate mofetil, but the abnormalities are usually mild, asymptomatic and resolve spontaneously or with dose reduction. A small number of cases of clinically apparent liver injury have been reported in patients on mycophenolate. The onset of injury is usually during the first month of therapy and the pattern of serum enzyme elevations is hepatocellular or mixed. The liver injury is usually mild and self-limiting. Autoimmune and immunoallergic features are uncommon.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Mycophenolate mofetil is a prodrug and undergoes extensive metabolism to mycophenolic acid which is pharmacologically active and undergoes enterohepatic recirculation. Mycophenolic acid is converted to a glucuronide by glucuronyl transferase and excreted largely in the urine. Idiosyncratic liver injury is likely due to a toxic or immunogenic metabolite. Mycophenolate does not seem to affect cytochrome P450 enzymes.
Outcome and Management
The liver injury due to mycophenolate is usually mild and self-limited, resolving by itself or responding rapidly to dose modification or discontinuation. Mycophenolate has not been linked to instances of acute liver failure or vanishing bile duct syndrome. There is no evidence of cross sensitivity to the hepatic injury between mycophenolate and other medications used to prevent rejection such as the calcineurin inhibitors or sirolimus.
Agents used specifically for the prophylaxis against allograft rejection include cyclosporine, mycophenolate mofetil, sirolimus and tacrolimus, as well as azathioprine and corticosteroids.
Drug Class: Transplant Agents; Antirheumatic Agents, Major Immunosuppressive Agents
Other Drugs in the Class, Transplant Agents: Cyclosporine, Sirolimus, Tacrolimus
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mycophenolate Mofetil – Generic, CellCept®
DRUG CLASS
Transplant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mycophenolate Mofetil | 128794-94-5 | C23-H31-N-O7 |
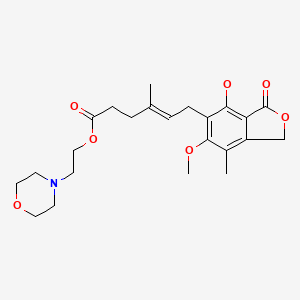
|
ANNOTATED BIBLIOGRAPHY
- References updated: 12 February 2020.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 does not mention or discuss mycophenolate).
- Reuben A. Hepatotoxicity of immunosuppressive drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 569-92.(Review of hepatotoxicity of immunosuppressive agents mentions that reports of hepatotoxicity of cyclosporine have decreased since the 1980s, perhaps because of monitoring of serum levels and lower doses used; liver injury from tacrolimus, sirolimus and mycophenolate is rare and usually rapidly reversible).
- Krensky AM, Azzi JR, Hafler DA. Immnosuppressants and tolerogens. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 637-54.(Textbook of pharmacology and therapeutics).
- Simmons WD, Rayhill SC, Sollinger HW. Preliminary risk-benefit assessment of mycophenolate mofetil in transplant rejection. Drug Saf. 1997;17:75–92. [PubMed: 9285199](Review of mechanism of action, pharmacology, clinical efficacy and toxicity of mycophenolate for transplant rejection; gastrointestinal intolerance was the most common side effect; no mention of effect on ALT levels or hepatotoxicity).
- Basara N, Fauser AA. Safety profile of mycophenolate mofetil. Bone Marrow Transplant. 2000;26:1362–3. [PubMed: 11223981](Letter stressing the good safety profile of mycophenolate and that severe liver injury after bone marrow transplantation is more likely due to graft-vs-host disease than drug induced liver injury).
- Corrieri-Baizeau C, Dumortier J, Scoazec JY, Poncet G, Choucair A, Vial T, Boillot O. Gastroenterol Clin Biol. 2002;26:300–1. [Mycophenolate mofetil induced acute hepatitis] French. [PubMed: 11981480](36 year old liver transplant recipient developed ALT elevations 5 times ULN 2 weeks after starting mycophenolate [bilirubin normal, Alk P 1.5 times ULN], biopsy not showing rejection and tests returning to normal 6 weeks after stopping mycophenolate).
- Sen HN, Suhler EB, Al-Khatib SQ, Djalilian AR, Nussenblatt RB, Buggage RR. Mycophenolate mofetil for the treatment of scleritis. Ophthalmology. 2003;110:1750–5. [PubMed: 13129873](One of 8 patients with scleritis treated with mycophenolate developed liver test abnormalities, but patient was also receiving methotrexate).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, 124 for acetaminophen and 137 for other drugs or toxins, but none for agents used to prevent transplant rejection).
- Balal M, Demir E, Paydas S, Sertdemir Y, Erken U. Uncommon side effect of MMF in renal transplant recipients. Ren Fail. 2005;27:591–4. [PubMed: 16152998](Among 79 renal transplant recipients receiving mycophenolate, 11 had serum ALT elevations [51-508 U/L] 4-70 days after transplant and resolving with lowering dose or stopping mycophenolate).
- Loupy A, Anglicheau D, Mamzer-Bruneel MF, Martinez F, Thervet E, Legendre C, Serpaggi J, et al. Mycophenolate sodium-induced hepatotoxicity: first report. Transplantation. 2006;82:581. [PubMed: 16926609](42 year old renal transplant recipient with chronic hepatitis C developed rash and jaundice 17 days after switching from sirolimus to mycophenolate [bilirubin 11.1 mg/dL, ALT 140 U/L], with no change in HCV RNA levels and resolution upon stopping mycophenolate).
- Jakab SS, West AB, Meighan DM, Brown RS Jr, Hale WB. Mycophenolate mofetil for drug-induced vanishing bile duct syndrome. World J Gastroenterol. 2007;13:6087–9. [PMC free article: PMC4250896] [PubMed: 18023105](69 year old man developed jaundice followed by vanishing bile duct syndrome 3 weeks after a course of amoxicillin/clavulanate, which appeared to improve upon corticosteroid and then mycophenolate therapy).
- Dourakis SP, Boki K, Soultati A, Cherouvim E, Delladetsima I. Acute hepatitis following mycophenolate mofetil administration for ANCA-positive vasculitis. Scand J Rheumatol. 2007;36:237–9. [PubMed: 17657683](73 year old man with systemic vasculitis developed abnormal liver tests 5 months after starting mycophenolate [bilirubin 1 mg/dL, ALT 750 U/L, Alk P 500 U/L], resolving 12 weeks after stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to cyclosporine, tacrolimus, sirolimus or mycophenolate).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to an agent used to prevent transplant rejection).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to an anti-rejection agent used in transplantation).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, one was attributed to mycophenolate but none to cyclosporine, sirolimus or tacrolimus).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to cyclosporine, but none to tacrolimus, sirolimus or mycophenolate mofetil).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Mycophenolate mofetil: a review of its use in the management of solid organ transplantation.[BioDrugs. 1999]Mycophenolate mofetil: a review of its use in the management of solid organ transplantation.Bardsley-Elliot A, Noble S, Foster RH. BioDrugs. 1999 Nov; 12(5):363-410.
- Review Voclosporin.[LiverTox: Clinical and Researc...]Review Voclosporin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Mycophenolate mofetil: a unique immunosuppressive agent.[Am J Health Syst Pharm. 1997]Review Mycophenolate mofetil: a unique immunosuppressive agent.Hood KA, Zarembski DG. Am J Health Syst Pharm. 1997 Feb 1; 54(3):285-94.
- Survival Associated With Sirolimus Plus Tacrolimus Maintenance Without Induction Therapy Compared With Standard Immunosuppression After Lung Transplant.[JAMA Netw Open. 2019]Survival Associated With Sirolimus Plus Tacrolimus Maintenance Without Induction Therapy Compared With Standard Immunosuppression After Lung Transplant.Wijesinha M, Hirshon JM, Terrin M, Magder L, Brown C, Stafford K, Iacono A. JAMA Netw Open. 2019 Aug 2; 2(8):e1910297. Epub 2019 Aug 2.
- Rescue therapy with tacrolimus and mycophenolate mofetil does not prevent deterioration of graft function in C4d-positive chronic allograft nephropathy.[Wien Klin Wochenschr. 2006]Rescue therapy with tacrolimus and mycophenolate mofetil does not prevent deterioration of graft function in C4d-positive chronic allograft nephropathy.Schwarz C, Regele H, Huttary N, Wahrmann M, Exner M, Nagy-Bojarsky K, Kletzmayr J, Hörl WH, Böhmig GA. Wien Klin Wochenschr. 2006 Jul; 118(13-14):397-404.
- Mycophenolate - LiverToxMycophenolate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...