NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Phenylbutyrate and sodium benzoate are orphan drugs approved for the treatment of hyperammonemia in patients with urea cycle disorders, a series of at least 8 rare genetic enzyme deficiencies. The urea cycle is the major pathway of elimination of excess nitrogen including ammonia, and absence of one of the urea cycle enzymes often causes elevations in serum ammonia which can be severe, life-threatening and result in permanent neurologic damage and cognitive deficiencies. Both phenylbutyrate and sodium benzoate act by promoting an alternative pathway of nitrogen elimination. Neither phenylbutyrate nor sodium benzoate have been linked to cases of liver injury either in the form of serum enzyme elevations during therapy or clinically apparent acute liver injury.
Phenylbutyrate
Background
Phenylbutyrate (fen" il beu' ti rate) is a prodrug that is metabolized to phenylacetate, which is the active molecule that combines with glutamine (an amino acid with two nitrogen molecules) to form phenylacetylglutamine, which is rapidly excreted by the kidneys and does not require metabolism via the urea cycle. Phenylbutyrate thus provides an “ammonia sink”, an alternative pathway for excretion of excess nitrogen and ammonia. The active metabolite phenylacetate is also effective therapeutically, but has a disagreeable odor and taste that affect compliance and acceptability. Phenylbutyrate is odorless but does have a bitter, salty taste and is better tolerated than phenylacetate, but still not well accepted, particularly because it must be given in high doses, often as 3 to 12 tablets three times daily. Nevertheless, phenylbutyrate has been found to be effective in lowering ammonia levels in newborns, children and adults with acute hyperammonemic crises as well as to maintain normal or near normal levels of ammonia in patients between episodes (sometimes brought on by infection or excess dietary protein). Sodium phenylbutyrate received orphan drug approval for this indication in 1996. It is available in tablets of 500 mg and as a powder for oral solution under the brand name Buphenyl. The typical dose varies by body weight or surface area, but is in general in the range of 5 to 20 grams daily given in three equally divided doses with meals. Phenylbutyrate is administered in conjunction with a low protein diet, often combined with sodium benzoate (another ammonia “sink”) and essential amino acids (such as citrulline or arginine). However, the regimen used must be individualized based upon the type of urea cycle disorder and specific clinical features. Phenylbutyrate should be administered only by physicians with expertise in managing urea cycle disorders and with proper diagnostic evaluation and monitoring. Common effects of sodium phenylbutyrate are bitter taste, loss of appetite, nausea, vomiting, diarrhea and edema. Rare side effects include fever and rash. Because of the need to calculate dosages, overdosing can easily occur. Accidental use of higher than appropriate doses of phenylbutyrate can result in severe metabolic side effects and death.
The poor acceptance of standard, sodium phenylbutyrate because of its bitter taste, high sodium content and pill burden (as many as 40 tablets daily) was a major impetus to the development of the glycerol-tri-phenylbutyrate, a formulation that is both tasteless and odorless, has a low sodium content and can be given orally as a liquid in a small volume. Glycerol phenylbutyrate was approved for treatment of hyperammonemia due to urea cycle disorders in 2013 and is available as an oral solution (1.1 g/mL) under the brand name Ravicti. The typical dose is 5 to 12 g/m2 daily (~5-10 mL) in three divided doses with meals. The common side effects of sodium phenylbutyrate such as bitter taste, anorexia, nausea and vomiting are less with glycerol phenylbutyrate and the high sodium intake of the standard formulation can be avoided. Nevertheless, care in calculation of the dose is critical, and monitoring of ammonia and drug levels during treatment is recommended.
Hepatotoxicity
While the urea cycle disorders are caused by deficiencies of hepatic enzymes responsible for the elimination of nitrogen, patients generally present with hyperammonemia without other features or biochemical evidence of hepatic injury. Thus, serum aminotransferase, alkaline phosphatase and bilirubin levels are generally normal or only mildly elevated. Newborns presenting with hyperammonemia may have hepatomegaly but other, non-urea cycle, liver function is normal as is hepatic histology. Phenylbutyrate can help to lower ammonia levels acutely and manage to keep them in the normal or near normal range, but generally does not affect other liver functions. In open label studies, a small proportion of patients (particularly with ornithine transcarbamylase [OTC] deficiency) have had ALT or AST elevations, but these have generally been attributed to the underlying condition or its complications. Phenylbutyrate has not been linked to instances of clinically apparent liver injury with jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury, but experience with its use is limited).
Sodium Benzoate
Introduction
Sodium benzoate was the first agent developed specifically for the therapy of hyperammonemia caused by urea cycle disorders. Like phenylbutyrate, sodium benzoate acts as an ammonia sink, eliminating nitrogen by an alternative pathways independent of the urea cycle. Sodium benzoate has not been linked to significant serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury.
Background
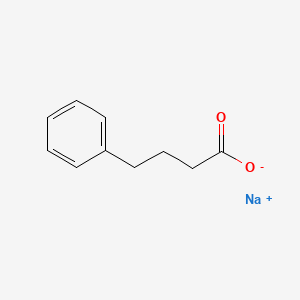
Sodium benzoate (ben' zoe ate) is a small molecule that conjugates with glycine (an amino acid with one nitrogen molecule) to form hippuric acid, which is rapidly excreted by the kidneys and does not require metabolism via the urea cycle. Sodium benzoate is an orphan drug that is approved for the treatment of hyperammonemia in patients with urea cycle disorders, a series of at least 8 rare genetic deficiencies of enzymes involved in the urea cycle and elimination of nitrogen waste. Sodium benzoate has been shown to result in a rapid decrease in serum ammonia levels in children and adults with urea cycle disorders. It is less effective than phenylbutyrate, perhaps because it conjugates to glycine which has a single nitrogen molecule as opposed to phenylbutyrate which conjugates to glutamate which possesses two nitrogens. Nevertheless, because sodium benzoate acts via a different amino acid than phenylbutyrate, the two drugs can be used in combination to treat refractory cases of hyperammonemia due to urea cycle disorders. Indeed, the combination of sodium benzoate with phenylbutyrate was approved for use in the United States in 1996 for the treatment of hyperammonemic crises in children and adults with urea cycle disorders. The combination of sodium benzoate and phenylacetate is available as a solution for injection in single dose vials (50 mL: 50 mg of each) generically and under the brand name Ammonul. Oral formulations of sodium benzoate are available in other countries of the world. The intravenous preparation is used only to treat hyperammonemic crises with encephalopathy and the dose varies by type of urea cycle disorder and body weight. The intravenous combination of sodium benzoate and phenyl-acetate or -butyrate should be administered only by a physician with expertise in the management of urea cycle disorders. The intravenous infusion should be given via a central and not peripheral line. Common effects of sodium benzoate are nausea, vomiting, injection site reactions, fever, and rash. Because this product is given to patients with severe hyperammonemia who are often critically ill, many of the reported adverse events are more likely due to the underlying conditions rather than the sodium benzoate and phenylbutyrate; they include cardiac arrhythmias, hypoglycemia, respiratory distress and failure, seizures, coma, clonus, liver failure and hyperammonemia.
Hepatotoxicity
While the urea cycle disorders are caused by deficiencies of hepatic enzymes responsible for the elimination of nitrogen, patients generally present with hyperammonemia without other features of hepatic injury. Thus, serum aminotransferase, alkaline phosphatase and bilirubin levels are generally normal or only mildly elevated. Newborns presenting with hyperammonemia may have hepatomegaly but other, non-urea cycle, liver function is normal as is hepatic histology. Sodium benzoate can help to lower ammonia levels acutely and manage to keep them in the normal or near normal range, but generally does not affect other liver functions. Transient and mild serum enzyme elevations have been described during therapy with sodium benzoate (with or without phenylbutyrate), but these are generally attributed to the underlying condition or its complications. Sodium benzoate has not been linked to instances of clinically apparent liver injury with jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury, but experience with its use is limited).
Drug Class: Genetic Disorder Agents, Urea Cycle Disorder Agents (Hyperammonemia)
Other drugs in the Subclass: Carglumic Acid, Lactulose, Rifaximin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Glycerol Phenylbutyrate – Ravicti®
Sodium Phenylbutyrate – Generic, Buphenyl®
Sodium Benzoate and Sodium Phenylacetate – Generic, Ammonul®
DRUG CLASS
Urea Cycle Disorder Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 10 October 2016
Abbreviations used for urea cycle enzyme deficiencies: ASL, argininosuccinate lyase (citrullinemia); ASS, argininosuccinate synthetase; CPS, carbamylphosphate synthase; OTC, ornithine transcarbamylase.
- Brusilow SW, Valle DL, Batshaw M. New pathways of nitrogen excretion in inborn errors of urea synthesis. Lancet 1979; 2 (8140): 452-4. [PubMed: 89510](Initial description of biochemical basis for use of arginine, citrulline and sodium benzoate for hyperammonemia caused by urea cycle disorders, which take advantage of alternative pathways of excretion of excess nitrogen including ammonia).
- Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK, Cann HM, et al. Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. N Engl J Med 1982; 306: 1387-92. [PubMed: 7078580](Among 26 infants with 4 different urea cycle disorders and severe hyperammonemia treated with activators of alternative pathways of nitrogen excretion including sodium benzoate, citrulline and arginine for 7 to 62 months, 22 survived and most had normal or near normal plasma ammonia levels and “no serious side effects [of sodium benzoate] have been observed”, and serum ALT levels which were monitored during therapy “were normal or near normal”).
- Brusilow SW, Danney M, Waber LJ, Batshaw M, Burton B, Levitsky L, Roth K, McKeethren C, Ward J. Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. N Engl J Med 1984; 310: 1630-4. [PubMed: 6427608](In 12 episodes of acute hyperammonemia occurring in 7 children with urea cycle disorders treated with a regimen of intravenous sodium benzoate, phenylacetate and arginine with nitrogen-free alimentation, 11 survived and “the only side effect of therapy was…nausea and vomiting with the priming infusion”).
- Finkelstein JE, Hauser ER, Leonard CO, Brusilow SW. Late-onset ornithine transcarbamylase deficiency in male patients. J Pediatr 1990; 117: 897-902. [PubMed: 2246687](Among 21 males with OTC deficiency presenting after 28 days of age, all appeared normal at birth and developed episodic irritability, vomiting and lethargy, and 12 survived and were maintained on sodium benzoate, phenylacetate or phenylbutyrate, overall acceptance with strict compliance being poor [~17%]).
- Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood 1994; 84: 339-43. [PubMed: 7517215](Among 6 patients with sickle cell disease [ages 13 to 46 years] who were treated with phenylbutyrate [13 g/m2 daily] for 8-43 days, all had an increase in fetal hemoglobin, but compliance was poor and adverse events included rash, sodium overload, edema and fever; no mention of ALT elevations or hepatotoxicity).
- Sodium phenylbutyrate for urea cycle enzyme deficiencies. Med Lett Drugs Ther 1996; 38 (988): 105-6. [PubMed: 8941257](Concise review of the mechanism of action, efficacy, side effects and costs of phenylbutyrate shortly after its approval for use in the urea cycle disorders in 1996; mentions that serum enzyme elevations have occurred during therapy, but are most likely due to the underlying disease).
- Maestri NE, Brusilow SW, Clissold DB, Bassett SS. Long-term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med 1996; 335: 855-9. [PubMed: 8778603](Among 39 girls with OTC deficiency and episodic hyperammonemia who were treated with phenylbutyrate and/or sodium benzoate, frequency of hyperammonemic crises and survival appeared to be better than that of historic controls; adverse events including ALT elevations were not mentioned).
- Feillet F, Leonard JV. Alternative pathway therapy for urea cycle disorders. J Inherit Metab Dis 1998; 21 Suppl 1: 101-11. [PubMed: 9686348](Review of the biochemistry of urea cycle disorders and rationale of use of agents that provide an alternative pathway for removal of nitrogen including sodium benzoate [glycine], phenylacetate and phenylbutyrate [glutamate] mentions that side effects in retrospective studies were difficult to attribute to therapy as opposed to the underlying disease).
- Saudubray JM, Touati G, Delonlay P, Jouvet P, Narcy C, Laurent J, Rabier D, et al. Liver transplantation in urea cycle disorders. Eur J Pediatr 1999; 158 Suppl 2: S55-9. [PubMed: 10603100](Retrospective analysis of 175 French subjects with urea cycle disorders, mainly OTC [119: 68%], CPS [13: 6%], ASS [citrullinemia 28: 37%] and ASL deficiency [15: 8%], comments upon the frequency of death or poor outcome and possible role of early liver transplantation to avert the serious neurologic and cognitive sequelae).
- Praphanphoj V, Boyadjiev SA, Waber LJ, Brusilow SW, Geraghty MT. Three cases of intravenous sodium benzoate and sodium phenylacetate toxicity occurring in the treatment of acute hyperammonaemia. J Inherit Metab Dis 2000; 23: 129-36. [PubMed: 10801054](Among 3 children [3-6 years old] with hyperammonemia due to urea cycle disorders who received inappropriate high doses of intravenous sodium benzoate and phenylacetate, all developed worsening somnolence, tachypnea and metabolic acidosis; two died and one survived after hemodialysis and with residual severe cognitive impairment).
- Bogdanovic MD, Kidd D, Briddon A, Duncan JS, Land JM. Late onset heterozygous ornithine transcarbamylase deficiency mimicking complex partial status epilepticus. J Neurol Neurosurg Psychiatry 2000; 69: 813-5. [PMC free article: PMC1737159] [PubMed: 11080238](A 57 year old woman developed hyperammonemic coma after receiving valproate for refractory seizures and was found to have OTC deficiency, responding to dietary protein restriction and chronic phenylbutyrate therapy).
- Redonnet-Vernhet I, Rouanet F, Pedespan JM, Hocke C, Parrot F. A successful pregnancy in a heterozygote for OTC deficiency treated with sodium phenylbutyrate. Neurology 2000; 54: 1008. [PubMed: 10691008](A 27 year old woman with OTC deficiency on long term therapy with phenylbutyrate and low protein diet delivered a normal infant at 33 weeks despite continuing low doses of phenylbutyrate during pregnancy).
- Berry GT, Steiner RD. Long-term management of patients with urea cycle disorders. J Pediatr 2001; 138 (1 Suppl): S56-60. [PubMed: 11148550](Brief summary of phenylbutyrate therapy and management of patients with urea cycle disorders stressing the need for careful monitoring and individualized therapy).
- Burlina AB, Ogier H, Korall H, Trefz FK. Long-term treatment with sodium phenylbutyrate in ornithine transcarbamylase-deficient patients. Mol Genet Metab 2001; 72: 351-5. [PubMed: 11286510](Among 9 children with OTC deficiency [4 boys, 5 girls] treated with phenylbutyrate for 17-42 months, ammonia levels were well controlled, there were no hyperammonemic episodes requiring hospitalization, and “no side effects related to therapy were observed”).
- Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr 2001; 138 (1 Suppl): S46-54; discussion S54-5. [PubMed: 11148549](Review of the history of development of alternative pathway therapy for urea cycle disorders including sodium benzoate and phenylbutyrate).
- Anadiotis G, Ierardi-Curto L, Kaplan PB, Berry GT. Ornithine transcarbamylase deficiency and pancreatitis. J Pediatr 2001; 138: 123-4. [PubMed: 11148526](A 15 year old boy with OTC deficiency, due to a rare mutation, developed pancreatitis while on phenylbutyrate and low protein diet given by gastrostomy tube [amylase 409 U/L, lipase 1172 U/L, ammonia 276 µmoL/L], followed by chronic amylase and lipase elevations, the cause being unclear; no information on whether phenylbutyrate was stopped).
- Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol 2002; 7: 27-35. [PubMed: 12069536](Review of the biochemical and genetic basis for the urea cycle disorders, their management and therapy including low protein diet, arginine, citrulline, sodium benzoate, phenylbutyrate, carnitine and dialysis).
- Gordon N. Ornithine transcarbamylase deficiency: a urea cycle defect. Eur J Paediatr Neurol 2003; 7: 115-21. [PubMed: 12788037](Review of OTC deficiency including use of sodium benzoate and phenylbutyrate; no discussion of side effects).
- Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, et al.; Urea Cycle Disorders Consortium of the Rare Diseases Clinical Research Network. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab 2008; 94: 397-402. [PMC free article: PMC2640937] [PubMed: 18562231](Among 183 patients with urea cycle disorders enrolled in a US consortium, causes included OTC deficiency [55%], ASA deficiency [16%] and citrullinemia [14%]; 63% were on a protein restricted diet, 37% phenylbutyrate and 5% sodium benzoate; no discussion of side effects).
- Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicenter study of acute hyperammonaemic episodes. Acta Paediatr 2008; 97: 1420-5. [PMC free article: PMC2675643] [PubMed: 18647279](Among 260 patients with urea cycle disorders presenting at four referral centers between 1982 and 2003, deficiencies included OTC [55%], ASS [27%], CPS [14%] and ASL [3%]; 985 hospitalizations for a hyperammonemic episode occurred, often following an acute, intercurrent illness [58%] or noncompliance [15%] and survival varied greatly by diagnosis and age of presentation).
- Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes Metab 2009 Sep; 11: 823-35. [PubMed: 19531057](Review of hyperammonemia and its treatment in urea cycle disorders including use of sodium benzoate and phenylbutyrate; mentions side effects of mucositis, but not ALT elevations or hepatotoxicity).
- McGuire BM, Zupanets IA, Lowe ME, Xiao X, Syplyviy VA, Monteleone J, Gargosky S, et al. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology 2010; 51: 2077-85. [PMC free article: PMC3733097] [PubMed: 20512995](Single dose, single-day and multiple-day dosing with glycerol phenylbutyrate in healthy adults and patients with cirrhosis found that the glycerol formulation achieved similar drug levels, had similar metabolism [with and without cirrhosis] and was not associated with serious adverse events or significant changes in liver test abnormalities).
- Lee B, Rhead W, Diaz GA, Scharschmidt BF, Mian A, Shchelochkov O, Marier JF, et al. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Mol Genet Metab 2010; 100: 221-8. [PMC free article: PMC2905228] [PubMed: 20382058](Among 10 adults with urea cycle disorders and episodic hyperammonemia who were switched from sodium phenylbutyrate to glycerol tri-phenylbutyrate, ammonia levels were better maintained and no hyperammonemic episodes occurred on the glycerol formulation which was better tolerated and accepted by patients).
- Cederbaum S, Lemons C, Batshaw ML. Alternative pathway or diversion therapy for urea cycle disorders now and in the future. Mol Genet Metab 2010; 100: 219-20. [PubMed: 20462778](Commentary on the publication by Lee [2010] reviewing the history of development of alternative pathway therapy of the urea cycle disorders, the difficulty of getting these agents approved and the promising new glycerol formulation of phenylbutyrate that promises to solve the problems of poor acceptance, high pill count and excess sodium intake associated with the standard formulation).
- Nagamani SC, Erez A, Lee B. Argininosuccinate lyase deficiency. Genet Med 2012; 14: 501-7. [PMC free article: PMC3709024] [PubMed: 22241104](Review of argininosuccinate lyase [ASL] deficiency, the second most frequent urea cycle disorder, its biochemical and genetic basis, clinical features, management, therapy and prognosis, therapy focusing upon low protein diet, sodium benzoate, phenylbutyrate and arginine replacement).
- Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 2011; 11: 227-49. [PMC free article: PMC3586072] [PubMed: 21902286](Discussion of the other possible, although still unproven, mechanisms of action and uses of phenylbutyrate including as a histone deacetylase inhibitor and drug chaperone [in cancer chemotherapy, sickle cell disease, cystic fibrosis and Huntington disease]).
- Lichter-Konecki U, Diaz GA, Merritt JL 2nd, Feigenbaum A, Jomphe C, Marier JF, Beliveau M, et al. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol Genet Metab 2011; 103: 323-9. [PMC free article: PMC4880058] [PubMed: 21612962](Among 11 patients with urea cycle disorders who were switched from sodium phenylbutyrate to glycerol-tri-phenylbutyrate, ammonia levels were lower with the new formulation which was also better accepted; no safety issues or coma episodes arose during therapy with the new formulation).
- Guffon N, Kibleur Y, Copalu W, Tissen C, Breitkreutz J. Developing a new formulation of sodium phenylbutyrate. Arch Dis Child 2012; 97: 1081-5. [PubMed: 22941860](Comparison of taste profile, tolerance and pharmacokinetics of standard vs glycerol phenylbutyrate in 13 healthy volunteers, found similar maximal plasma levels and bioequivalence, but no bitter taste and better acceptability of the glycerol formulation).
- Guha M. Urea cycle disorder drug approved. Nat Biotechnol 2013; 31: 274. [PubMed: 23563410](News article announcing the FDA approval of glycerol phenylbutyrate for urea cycle disorders which is likely to replace standard sodium phenylbutyrate because of its heavy pill count, high sodium content and bitter taste).
- Smith W, Diaz GA, Lichter-Konecki U, Berry SA, Harding CO, McCandless SE, LeMons C, et al. Ammonia control in children ages 2 months through 5 years with urea cycle disorders: comparison of sodium phenylbutyrate and glycerol phenylbutyrate. J Pediatr 2013; 162: 1228-34. [PMC free article: PMC4017326] [PubMed: 23324524](Among 15 children with urea cycle disorders who were switched from standard to glycerol-phenylbutyrate in an open label study, the glycerol formulation was non-inferior in controlling of ammonia levels and had better acceptability; there were no serious adverse events or changes in serum chemistry measures during dosing).
- Lamb S, Aye CY, Murphy E, Mackillop L. Multidisciplinary management of ornithine transcarbamylase (OTC) deficiency in pregnancy: essential to prevent hyperammonemic complications. BMJ Case Rep 2013; 2013. [PMC free article: PMC3604418] [PubMed: 23283608](A 29 year old woman with OTC deficiency was managed during a successful pregnancy with a resultant normal neonate, but the details or diet, sodium benzoate, phenylbutyrate and arginine doses were not provided).
- Diaz GA, Krivitzky LS, Mokhtarani M, Rhead W, Bartley J, Feigenbaum A, Longo N, et al. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology 2013; 57: 2171-9. [PMC free article: PMC3557606] [PubMed: 22961727](Analyses of a pivotal trial, short term studies of ammonia control and of long term open label studies using glycerol tri-phenylbutyrate reported similar control of ammonia levels compared to standard phenylbutyrate, but better acceptability; there were “no clinically significant laboratory” changes but common adverse events included nausea and vomiting, diarrhea, headache and decreased appetite, but no instances of clinically apparent liver injury besides hyperammonemia).
- Mistry PK. Rare disease clinical research network's urea cycle consortium delivers a successful clinical trial to improve alternate pathway therapy. Hepatology 2013; 57: 2100-2. [PubMed: 23080135](Editorial accompanying article by Diaz [2013] summarizing the history of development of alternate pathway therapy for the urea cycle disorders and the important role of a better accepted formulation of phenylbutyrate).
- Kibleur Y, Dobbelaere D, Barth M, Brassier A, Guffon N. Results from a Nationwide Cohort Temporary Utilization Authorization (ATU) survey of patients in France treated with Pheburane(®) (Sodium Phenylbutyrate) taste-masked granules. Paediatr Drugs 2014; 16: 407-15. [PMC free article: PMC4168023] [PubMed: 24962711](Among 25 patients with urea cycle disorders in France treated with taste-masked granules of phenylbutyrate [mean dose 5.2 g or 211 mg/kg daily] on a compassionate use basis for 1-11 months while the agent was being evaluated for approval, none had a hyperammonemic episode and “no patient reported any adverse event”).
- Berry SA, Lichter-Konecki U, Diaz GA, McCandless SE, Rhead W, Smith W, Lemons C, et al. Glycerol phenylbutyrate treatment in children with urea cycle disorders: pooled analysis of short and long-term ammonia control and outcomes. Mol Genet Metab 2014; 112: 17-24. [PMC free article: PMC4382922] [PubMed: 24630270](Pooled analysis of 2 studies of glycerol vs sodium phenylbutyrate showed that ammonia levels were better controlled with the glycerol formulation which also had better acceptability).
- Batshaw ML, Tuchman M, Summar M, Seminara J; Members of the Urea Cycle Disorders Consortium. A longitudinal study of urea cycle disorders. Mol Genet Metab 2014; 113 (1-2): 127-30. [PMC free article: PMC4178008] [PubMed: 25135652](Overview of the Urea Cycle Disorders Consortium and its ongoing clinical studies including a longitudinal analysis showing that phenylbutyrate therapy was associated with lower plasma levels of branched chained amino acids and another analysis showing that liver dysfunction was not uncommon in patients with OTC deficiency).
- Glycerol phenylbutyrate (Ravicti) for urea cycle disorders. Med Lett Drugs Ther 2014; 56 (1449): 77-8. [PubMed: 25118801](Concise review of the mechanism of action, clinical efficacy, safety and costs of glycerol phenylbutyrate as therapy of hyperammonemia in the urea cycle disorders, mentions that common adverse effects include diarrhea, flatulence and headache; no mention of ALT elevations or hepatotoxicity).
- Martín-Hernández E, Aldámiz-Echevarría L, Castejón-Ponce E, Pedrón-Giner C, Couce ML, Serrano-Nieto J, Pintos-Morell G, et al. Urea cycle disorders in Spain: an observational, cross-sectional and multicentric study of 104 cases. Orphanet J Rare Dis 2014; 9: 187. [PMC free article: PMC4258263] [PubMed: 25433810](Cross-sectional analysis of 104 patients with urea cycle disorders from 98 families and 21 centers in Spain found most common form was ornithine transcarbamylase deficiency [64%], followed by type 1 citrullinemia [21%] and argininosuccinic aciduria [10%]; most were treated with essential amino acid replacement, some with sodium benzoate, phenylbutyrate and one with carglumic acid [with NAGS deficiency]).
- Matoori S, Leroux JC. Recent advances in the treatment of hyperammonemia. Adv Drug Deliv Rev 2015; 90: 55-68. [PubMed: 25895618](Review of the causes and treatments of hyperammonemia focusing upon newer agents such as rifaximin, sodium benzoate, carglumic acid and phenylbutyrate).
- Lee B, Diaz GA, Rhead W, Lichter-Konecki U, Feigenbaum A, Berry SA, Le Mons C, et al. Glutamine and hyperammonemic crises in patients with urea cycle disorders. Mol Genet Metab 2016; 117: 27-32. [PMC free article: PMC4915945] [PubMed: 26586473](In longitudinal studies among 100 patients with urea cycle disorders in clinical trials of glycerol phenylbutyrate, plasma arginine showed less variability than ammonia levels, but was less reliable in predicting episodes of severe hyperammonemia).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: patient perspectives.[Patient Prefer Adherence. 2017]Review Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: patient perspectives.Peña-Quintana L, Llarena M, Reyes-Suárez D, Aldámiz-Echevarria L. Patient Prefer Adherence. 2017; 11:1489-1496. Epub 2017 Sep 6.
- Efficacy of orally administered sodium benzoate and sodium phenylbutyrate in dogs with congenital portosystemic shunts.[J Vet Intern Med. 2019]Efficacy of orally administered sodium benzoate and sodium phenylbutyrate in dogs with congenital portosystemic shunts.van Straten G, van Dalen D, Mesu SJ, Rothuizen J, Teske E, Spee B, Favier RP, van Geijlswijk IM. J Vet Intern Med. 2019 May; 33(3):1331-1335. Epub 2019 Mar 27.
- Review Ammonia toxicity and its prevention in inherited defects of the urea cycle.[Diabetes Obes Metab. 2009]Review Ammonia toxicity and its prevention in inherited defects of the urea cycle.Walker V. Diabetes Obes Metab. 2009 Sep; 11(9):823-35. Epub 2009 Jun 16.
- Review An update on the use of benzoate, phenylacetate and phenylbutyrate ammonia scavengers for interrogating and modifying liver nitrogen metabolism and its implications in urea cycle disorders and liver disease.[Expert Opin Drug Metab Toxicol...]Review An update on the use of benzoate, phenylacetate and phenylbutyrate ammonia scavengers for interrogating and modifying liver nitrogen metabolism and its implications in urea cycle disorders and liver disease.De Las Heras J, Aldámiz-Echevarría L, Martínez-Chantar ML, Delgado TC. Expert Opin Drug Metab Toxicol. 2017 Apr; 13(4):439-448. Epub 2016 Nov 28.
- Review Recent advances in the treatment of hyperammonemia.[Adv Drug Deliv Rev. 2015]Review Recent advances in the treatment of hyperammonemia.Matoori S, Leroux JC. Adv Drug Deliv Rev. 2015 Aug 1; 90:55-68. Epub 2015 Apr 17.
- Phenylbutyrate, Sodium Benzoate - LiverToxPhenylbutyrate, Sodium Benzoate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...