NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Penicillin G and V are first generation penicillins that are used widely to treat infections due to susceptible organisms and have been linked rarely and only weakly with idiosyncratic liver injury.
Background
Penicillin G benzathine, potassium, procaine and sodium are currently available in the United States in parenteral formulations for intravenous or intramuscular use. Penicillin V potassium (also called phenoxymethyl penicillin) is a more acid stable and can be administered orally. Both Penicillin G and V are available in multiple generic formulations. The natural penicillins are indicated as therapy for mild-to-severe infections caused by susceptible organisms including (but not limited to) streptococcal infections and pneumonia, enterococcal and non-enterococcal endocarditis, diphtheria, anthrax, bacterial meningitis, Lyme disease, gonorrhea, syphilis, actinomycosis, botulism and others. The first generation penicillins are susceptible to inactivation by beta-lactamase, and resistance is relatively common. The usual doses of intravenous penicillin G are 300,000 to 4 million units every 6 to 8 hours. Benzathine penicillin G is given in a one time dose of 2.4 million units intramuscularly as therapy of primary or secondary syphilis, and as three weekly doses for syphilis of longer duration; it is also used as prophylaxis against rheumatic fever. Penicillin V is available in tablets of 250 and 500 mg and is usually given in doses of 250 to 500 mg every 6 to 8 hours for 7 to 20 days. It is also available as an oral solution. Side effects of penicillin G and V include nausea, diarrhea, gastrointestinal upset, headache, dizziness, rash and hypersensitivity reactions. Severe adverse events include anaphylaxis which can be severe and even fatal, angioedema, pseudomembranous colitis, C. difficile infection and Jarisch-Herxheimer reactions.
Hepatotoxicity
Rare instances of idiosyncratic liver injury have been reported in persons receiving the first generation penicillins. Many case reports predated availability of serologic testing for viral hepatitis and many described patients with multiple reasons for having liver disease (such as sepsis) and who were receiving other potentially hepatotoxic agents. Three distinct forms of liver injury can occur with the first generation penicillins: (1) transient, asymptomatic elevations in serum aminotransferase levels with prolonged high doses of parenteral penicillin, (2) minor liver injury associated with severe hypersensitivity reactions, and (3) idiosyncratic, delayed cholestatic hepatitis. These three forms of injury probably occur with all four generations of penicillin, some being more common with one form of penicillin than another.
High doses of intravenous and intramuscular penicillin can be associated with serum aminotransferase elevations that are usually asymptomatic and resolve rapidly with stopping therapy or switching to another antibiotic (Case 1). Jaundice and elevations in alkaline phosphatase are usually absent or mild. This type of hepatotoxicity is most common with oxacillin and carbenicillin, but can occur with parenteral forms of the first generation penicillins as well. This form of injury appears to be direct hepatotoxicity.
Patients with severe hypersensitivity reactions to penicillin, such as Stevens-Johnson syndrome or anaphylaxis, may have an accompanying liver injury and jaundice, but it is not clear whether this represents true penicillin hepatotoxicity or a complication of hyperthermia, shock and generalized immune reactivity. Generalized allergic reactions to penicillin may be accompanied by granulomas in the liver, spleen and kidney, but are usually without evidence of specific hepatitis injury. Virtually all of the penicillins are associated with hypersensitivity reactions, but liver injury is usually overshadowed by the allergic complications (rash, fever, anaphylaxis).
Finally, isolated case reports have shown that the first generation penicillins can cause a delayed cholestatic hepatitis that probably represents idiosyncratic hepatotoxicity. Symptoms of nausea, abdominal discomfort, jaundice and pruritus generally arise 1 to 4 weeks after starting therapy, and often a few days or weeks after completing a course. The serum enzyme pattern is usually cholestatic, but may be mixed or hepatocellular if tested soon after onset. Immunoallergic features are common, but autoantibody formation is rare. Most cases are mild-to-moderate in severity and resolve rapidly (Case 2). This delayed form of idiosyncratic cholestatic hepatitis is typical of many penicillins and cephalosporins, varying in frequency with the specific form. Idiosyncratic, cholestatic hepatitis is quite rare with the natural penicillins, more common with certain broad spectrum penicillins (cloxacillin, flucloxacillin) and is most common with amoxicillin with clavulanic acid.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the idiosyncratic, cholestatic liver injury associated with penicillin is probably hypersensitivity or allergy. No cases of rechallenge or reexposure have been reported. The serum aminotransferase elevations that occur with high doses of parenteral penicillin are likely due to direct hepatotoxicity.
Outcome and Management
The asymptomatic rise in serum aminotransferase levels that occurs with high dose penicillin therapy usually resolves rapidly once penicillin is stopped. These patients may tolerate another form of penicillin without recurrence. In the few cases of cholestatic hepatitis that have been described with the first generation penicillins, patients have recovered although recovery was slow in some instances (2 to 6 months). Fatal cases of penicillin associated liver injury have been described, but usually in association with severe allergic reactions such as Stevens-Johnson syndrome in which shock and ischemic hepatitis may have contributed to the outcome. Patients with idiosyncratic liver injury attributed to penicillin should not be reexposed to other penicillins and given cephalosporins only with careful monitoring.
Drug Class: Antiinfective Agents, Penicillins (First Generation)
CASE REPORTS
Case 1. Serum aminotransferase elevations attributed to penicillin therapy.(1)
A 54 year old man with pyogenic vertebral spondylitis developed rising serum aminotransferase levels approximately 3 weeks after starting a course of high dose intravenous benzylpenicillin. On admission, and before starting penicillin, he was febrile and complained of fatigue and low back pain. He had no history of alcohol abuse or previous allergic reactions to medications or liver disease. Serum ALT was elevated (Table), but levels fell into the normal range as the infection came under control with antibiotic therapy. After 4 weeks of penicillin therapy, serum ALT levels were found to be elevated in association with marked eosinophilia, but the patient had no symptoms of liver disease and was not jaundiced. Penicillin was continued for another two weeks, but when ALT levels continued to rise antibiotic therapy was changed to a cephalosporin. Serum aminotransferase levels and eosinophilia promptly resolved. Tests for hepatitis A, B and C were negative as were autoantibodies. The patient received several other medications and underwent minor surgery under propofol and alfentanil anesthesia a few days before the onset of the marked ALT elevations. In follow up, he recovered from the infection and had normal liver tests and repeat viral serology was negative.
Key Points
| Medication: | Penicillin G benzathine (5 MU iv every 6 hours) |
|---|---|
| Pattern: | Hepatocellular (no alkaline phosphatase elevations) |
| Severity: | 1+ (serum enzyme elevations without jaundice) |
| Latency: | 3 weeks |
| Recovery: | Somewhat more than 3 weeks |
| Other medications: | Ibuprofen, ranitidine, low molecular weight heparin, chloral hydrate, diazepam and, on day 21, propofol and alfentanil anesthesia. |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P (U/L) | Bilirubin (mg/dL)** | Other |
|---|---|---|---|---|---|
| Pre | Pre | 106 | 159 | 0.6 | 0.2% eosinophils |
| Benzylpenicillin (5 MU iv q 6 hr) started for Streptococcal pyogenic vertebral spondylitis | |||||
| 5 days | 40 | ||||
| 11 days | 30 | Normal | |||
| 17 days | 25 | ||||
| 21 days | 25 | ||||
| General anesthesia with propofol and alfentanil for minor surgery (~ day 26) | |||||
| 29 days | 423 | 101 | 0.5 | 13% eosinophils | |
| 32 days | 570 | ||||
| 35 days | 610 | ||||
| 40 days | 0 | 680 | Penicillin stopped, ceftriaxone started | ||
| 45 days | 4 days | 600 | |||
| 48 days | 7 days | 400 | |||
| 56 days | 15 days | 230 | |||
| 2 months | 1 month | 160 | |||
| Normal Values | <37 | <109 | <1.2 | ||
- *
Estimates made from Figure 1.
- **
Bilirubin converted from µmol (1 mg/dL=17.1 µmol/L).
Comment
The asymptomatic rise in serum aminotransferase levels after 4 weeks of high dose penicillin therapy and their fall once it was stopped is suggestive of penicillin induced direct hepatic injury. The onset and pattern of hepatic injury resembled the common hepatotoxicity of oxacillin with asymptomatic rises in aminotransferases that decrease rapidly when stopped and are often associated with eosinophilia. In the current case, other diagnoses could not be completely ruled out such as drug induced liver disease from other medications (low molecular weight heparin) or an underlying condition that led to the baseline, pre-penicillin ALT elevations.
Case 2. Cholestatic hepatitis attributed to penicillin therapy.(2)
A 19 year old man took one tablet of penicillin for a sore throat and developed headache and nausea followed over the next two days by fever and jaundice. He had received two injections of Penicillin G one month previously without incident. He denied previous history of liver disease or penicillin allergy and had no risk factors for viral hepatitis. On admission to the hospital, he was febrile, jaundiced and had a generalized skin rash. He had tender hepatomegaly and splenic enlargement as well as cervical adenopathy. Blood tests showed elevations in serum bilirubin, but mild increases in serum enzymes. He recovered rapidly from the symptoms of fever and prostration, but remained jaundiced for several months. Three liver biopsies were performed; the initial biopsy showed cholestasis with moderate lymphocyte and eosinophil infiltrations; the follow up biopsies showed gradual improvement. During follow up, his serum bilirubin levels were intermittently elevated and alkaline phosphatase levels were minimally increased, but he was asymptomatic, afebrile and had normal ALT levels.
Key Points
| Medication: | Oral penicillin (200,000 U) |
|---|---|
| Pattern: | Mixed (R=2.1) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 2 days |
| Recovery: | More than 3 months |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| Took a single table of penicillin for sore throat | |||||
| 4 days | 3 days | 96 | 105 | 7.3 | Fever to 40 oC |
| 8 days | 7 days | 48 | 82 | 3.6 | |
| 25 days | 24 days | 31 | 51 | 3.1 | |
| 2 months | 2 months | 17 | 58 | 1.9 | |
| 3 months | 3 months | 9 | 56 | 0.8 | |
| 5 months | 5 months | 9 | 69 | 0.6 | |
| 11 months | 11 months | 9 | 68 | 1.9 | |
| Normal Values | 1-17 | 10-40 | <1.2 | ||
Comment
A dramatic example of immunoallergic, cholestatic hepatitis arising within days of taking a single dose of penicillin. The pattern of serum enzyme elevations and liver histology was fully supportive of a drug induced liver injury and there was no evidence of other forms of liver disease (although no imaging of the gallbladder was mentioned). The onset with fever and rash suggests hypersensitivity and are typical if not universal with penicillin associated acute liver injury. The short latency (4 days) is typical of cases with reexposure to the responsible agent; with first exposure, the latency is typically 2 to 4 weeks.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Various Generic
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Penicillin G (Benzylpenicillin) | 61-33-6 | C16-H18-N2-O4-S |
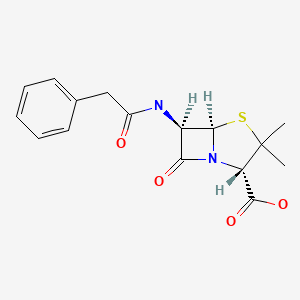
|
| Penicillin G Sodium | 69-57-8 | C16-H18-N2-O4-S |
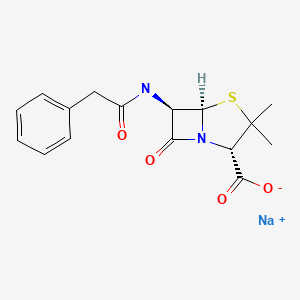
|
| Penicillin G Potassium | 113-98-4 | C16-H18-N2-O4-S.K |
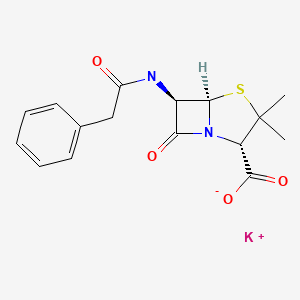
|
| Penicillin V | 87-08-1 | C16-H18-N2-O5-S |
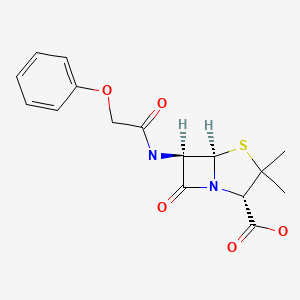
|
| Penicillin V Potassium | 132-98-9 | C16-H17-K-N2-O5-S C16-H17-N2-O5-S.K C16-H18-N2-O5-S.K |
|
CITED REFERENCES
- 1.
- Bauer TM, Bircher AJ. Drug-induced hepatocellular liver injury due to benzylpenicillin with evidence of lymphocyte sensitization. J Hepatol. 1997;26:429–32. [PubMed: 9059967]
- 2.
- Girard JP, Haenni B, Bergoz R, Kapanci Y, Cruchaud A. Lupoid hepatitis following administration of penicillin. Case report and immunological studies. Helv Med Acta. 1967;34:23–35. [PubMed: 4873914]
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; liver injury from penicillin is rare and usually associated with allergic reactions).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that liver injury from the natural penicillins is rare, with both hepatocellular and cholestatic patterns being described).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Howells L, Kerns JDO. Hepatitis after penicillin injection. Lancet. 1946;1:51–2. [PubMed: 21010116](Outbreak of hepatitis after penicillin injections at venereal disease centers, arising 62 to 157 days afterwards with jaundice lasting 10-44 days; "As a routine each syringe is used for about 5 patients, the syringe being boiled frequently, but not necessarily between each case"; an unfortunate example of iatrogenic spread of hepatitis B).
- Rabinovitch J, Snitkoff M. Acute exfoliative dermatitis and death following penicillin therapy. J Am Med Assoc. 1948;138:496–8. [PubMed: 18884886](72 year old woman developed generalized pruritus 7 days after completing an 11 day course of penicillin with fever, confluent exfoliative rash, progressive abdominal pain, pneumonia, jaundice, coma and death; difficult to say whether jaundice was due to penicillin reaction vs vascular collapse and sepsis).
- Felder SL, Felder L. Unusual reaction to penicillin. J Am Med Assoc. 1950;143:361–2. [PubMed: 15415270](53 year old man developed rash 10 days after starting intramuscular procaine penicillin with fever, giant urticaria, lymphadenopathy and Alk P 3 times ULN, but no jaundice, resolving within 2 weeks of stopping with conservative therapy using epinephrine and antihistamines).
- Waugh D. Myocarditis, arteritis, and focal hepatic, splenic, and renal granulomas apparently due to penicillin sensitivity. Am J Pathol. 1952;28:437–47. [PMC free article: PMC1937344] [PubMed: 14923834](60 year old man with multiple medical problems developed fever, eosinophilia and rash within hours of a penicillin injection requiring corticosteroids; sudden unexplained death 13 days later allowed for autopsy that showed interstitial myocarditis and arteritis with granulomas in liver, kidney and spleen, but no hepatitis or cholestasis).
- Murphy ES, Mireles M. Shock, liver necrosis, and death after penicillin injection. Arch Pathol. 1962;73:355–62. [PubMed: 14477414](66 year old woman with known penicillin allergy, given penicillin after surgery developed anaphylaxis with shock and diffuse bleeding, developing jaundice and dying several days later; autopsy showed centrolobular necrosis; unclear whether jaundice due to penicillin hepatotoxicity or ischemic liver injury, the autopsy favoring the later).
- Vardivia-Barriga V, Feldman A, Orellana J. Generalized hypersensitivity with hepatitis and jaundice after the use of penicillin and streptomycin. Gastroenterology. 1963;45:114–7. [PubMed: 14046304](25 year old woman had acute allergic reaction after an injection of penicillin and streptomycin becoming jaundiced within 2 days; other diagnoses could not be excluded in this early case).
- Girard JP, Haenni B, Bergoz R, Kapanci Y, Cruchaud A. Lupoid hepatitis following administration of penicillin. Case report and immunological studies. Helv Med Acta. 1967;34:23–35. [PubMed: 4873914](19 year old man developed fever, nausea and abdominal pain a few hours after a single dose of oral penicillin with jaundice arising 2 days later with prolonged course, many in vitro assays for penicillin allergy and three liver biopsies done; clearly not “lupoid” hepatitis, but reasonably convincing case of drug induced prolonged cholestasis and possibly vanishing bile duct syndrome: Case 2).
- Goldstein LI, Ishak KG. Hepatic injury associated with penicillin therapy. Arch Pathol. 1974;98:114–7. [PubMed: 4366005](Often cited paper describes 42 year old man who developed fever, arthralgias and mild alkaline phosphatase elevations [3 times ULN] with normal bilirubin [0.7 mg/dL] and ALT [40 U/L] 8 days after starting penicillin; a liver biopsy showed lobular unrest and mild hepatocyte injury).
- Beeley L, Gourevitch A, Kendall MJ. Jaundice after oral penicillin. Lancet. 1976;2:1297. [PubMed: 63763](Letter describing 36 year old woman who developed fever, rash and prostration after 48 hours of penicillin V [250 mg four times a day] with bilirubin 6.1 mg/dL, AST 62 U/L, alkaline phosphatase 40 U/L, resolving within 3 weeks).
- Paine TF Jr. Updating the side effects of the penicillins. Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1978;11:104–9. [PubMed: 581569](Review article mentions liver injury with high dose intravenous penicillins).
- Williams CN, Malatjalian DA. Severe penicillin-induced cholestasis in a 91-year-old woman. Dig Dis Sci. 1981;26:470–3. [PubMed: 7249889](91 year old woman received cloxacillin for 9 days followed by penicillin G for 5 days, developing symptoms by day 3 with subsequent cholestatic hepatitis, cloxacillin being the more likely culprit).
- Roberts J, Bianco MM, Fine J. Fatal anaphylactic reaction to oral penicillin: report of case. J Am Dent Assoc. 1985;110:505–6. [PubMed: 3923075](30 year old woman developed anaphylaxis and cardiopulmonary arrest within few minutes of single dose of Pen V; attempts at resuscitation failed; no mention of liver injury. Penicillin accounts for 75% of anaphylactic deaths, 400-800/year, and anaphylaxis incidence is 1-4/1000 exposures).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. [PubMed: 1506809](Adverse drug reaction reports in Denmark between 1978 and 1987; no mention of penicillins).
- Oñate J, Montejo M, Aguirrebengoa K, Ruiz-Irastorza G, González de Zárate P, Aguirre C. Hepatotoxicity associated with penicillin V therapy. Clin Infect Dis. 1995;20:474–5. [PubMed: 7742464](67 year old woman with actinomycosis was given penicillin G and then penicillin V; 10 days after starting she developed abdominal pain and at 15 days had abnormal liver tests [bilirubin 1.3 mg/dL, ALT 3073 U/L, Alk P normal], resolving after being switched to erythromycin).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Adverse drug reaction reports identified 943 cases of liver injury over 21 year period in New Zealand; no mention of penicillin).
- Bauer TM, Bircher AJ. Drug-induced hepatocellular liver injury due to benzylpenicillin with evidence of lymphocyte sensitization. J Hepatol. 1997;26:429–32. [PubMed: 9059967](54 year old man with osteomyelitis was started on benzylpenicillin [5 MU intravenously every 6 hours] and after 21 days developed eosinophilia and elevated liver tests [bilirubin 0.5 mg/dL, ALT 423 U/L, Alk P 101 U/L], resolving with stopping penicillin; on follow up he had positive lymphocyte stimulation test to benzylpenicillin, negative to amoxicillin and cephalosporin).
- Andrade RJ, Guilarte J, Salmerón FJ, Lucena MI, Bellot V. Benzylpenicillin-induced prolonged cholestasis. Ann Pharmacother. 2001;35:783–4. [PubMed: 11409000](28 year old woman with Streptococcal pharyngitis was given a single intramuscular injection of benzylpenicillin and developed abdominal pain, fever and jaundice 5 days later [bilirubin 7.6 mg/dL, ALT 357 U/L, Alk P 844 U/L, 1% eosinophils]; jaundice resolved rapidly, but liver enzymes were elevated for 18 months).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; one case was attributed to Penicillin V, but no details given).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, antimicrobials accounted for 45% of cases with 23 single agent cases due to amoxicillin/clavulanate, 13 nitrofurantoin, 10 fluoroquinolones, 9 macrolides, 9 sulfonamides, 5 cephalosporins, 3 oxacillin, 2 doxycycline, 2 amoxicillin, and one each for gentamicin, imipenem, and clindamycin, but none from first generation penicillins).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 66 due to antimicrobial agents, but only two due to amoxicillin and none attributed to natural penicillins).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: pathomechanisms and clinical data. Infection. 2010;38:3–11. [PubMed: 20107858](Review of mechanisms of liver injury due to antibacterial agents; first generation penicillins are not discussed).
- Ferrajolo C, Capuano A, Verhamme KMC, Schuemie M, Rossi F, Stricker BH, Sturkenboom CJM. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse drug reports in children in a worldwide pharmacovigilance database, 6595 [1%] were for hepatic injury and antibacterials accounted for 11%, those with the highest adjusted odds ratios being aztreonam, erythromycin, ceftriaxone and minocycline; no mention of penicillins).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin/clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric; none were attributed to a 4th generation penicillin).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 persons with suspected drug induced liver injury seen over at 12 month period at a referral hospital in Tasmania, 11 were attributed to antibiotics including 4 to flucloxacillin, 2 amoxicillin with clavulanate, 2 amoxicillin, and 1 each to rifampin, moxifloxacin and ciprofloxacin; none to 1st generation penicillins).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin/clavulanate).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to 1st, three to 2nd [all due to oxacillin], 97 to 3rd [91 to amoxicillin/clavulanate, and 6 to amoxicillin alone] and five to 4th generation penicillins [all 5 to piperacillin/tazobactam]).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses, but they did not apply to cephalosporin cases).
- Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. [PMC free article: PMC4783956] [PubMed: 26861310](Review of the most common causes of drug induced liver injury based upon categorization from LiverTox, amoxicillin/clavulanate but not other forms of penicillin were in the top category of causes of liver injury [likelihood scores of A, with more than 100 cases published in the literature]).
- Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients who underwent outpatient parenteral antibiotic therapy for at least 2 weeks, 210 [25%] developed eosinophilia including 58 of 207 [28%] who received “penicillins” of whom 3 developed signs of “possible” DRESS syndrome; specific penicillins accounting for the cases were not provided).
- Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. [PubMed: 26614821](Review of mechanisms of idiosyncratic drug induced liver injury focusing upon chemically reactive drug metabolites and genetic associations, particularly those with HLA alleles that implicate the adaptive immune response).
- Wilkinson J, Zainal A, Naqvi SY. Penicillin-induced liver injury during treatment for ocular neurosyphilis. BMJ Case Rep. 2016;2016:bcr2016215821. [PMC free article: PMC4956965] [PubMed: 27389728](51 year old man with ocular neurosyphilis developed fever and fatigue 10 days after starting a regimen of 60 mg of prednisone and 24 million units of iv penicillin G daily [bilirubin 1.2 rising to 2.3 mg/dL, ALT 1743 to 2727 U/L, Alk P 68 U/L, INR 1.2], improving rapidly once penicillin was discontinued).
- Takeuchi Y, Shinozaki T, Kumamaru H, Hiramatsu T, Matsuyama Y. Analyzing intent-to-treat and per-protocol effects on safety outcomes using a medical information database: an application to the risk assessment of antibiotic-induced liver injury. Expert Opin Drug Saf. 2018;17:1071–9. [PubMed: 30252549](Cohort matching of cases with vs without antibiotic therapy in a large electronic medical record database from the University of Tokyo Hospital from 2011 to 2015 with adjustments, found rates of liver test abnormalities within 30 days of starting penicillins [25.2 per 1000] were higher than that of fluoroquinolones [11.4] and macrolide antibiotics [8.1] as well as controls [6.5 to 7.1]).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Penicillins (3rd Generation).[LiverTox: Clinical and Researc...]Review Penicillins (3rd Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Penicillins (4th Generation).[LiverTox: Clinical and Researc...]Review Penicillins (4th Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Comparison of penicillins (penicillin G and ampicillin) and cefazolin as a definitive therapy against penicillin-susceptible Staphylococcus aureus (PSSA) bacteremia in Japan: a retrospective cohort study.[J Infect Chemother. 2020]Comparison of penicillins (penicillin G and ampicillin) and cefazolin as a definitive therapy against penicillin-susceptible Staphylococcus aureus (PSSA) bacteremia in Japan: a retrospective cohort study.Moriyama Y, Ishikane M, Mezaki K, Ohmagari N. J Infect Chemother. 2020 Apr; 26(4):358-362. Epub 2019 Dec 9.
- The newer penicillins.[Calif Med. 1962]The newer penicillins.SIMON HJ. Calif Med. 1962 Sep; 97(3):135-41.
- [INDICATIONS AND LIMITATIONS OF USE OF ORAL PENICILLINS].[Rev Prat. 1963][INDICATIONS AND LIMITATIONS OF USE OF ORAL PENICILLINS].MOZZICONACCI P, ATTAL C. Rev Prat. 1963 Dec 9; 13:SUPPL 124-7.
- Penicillin G and V - LiverToxPenicillin G and V - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...