NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Penicillamine is chelating agent used to decrease copper stores in Wilson disease, which also has immunomodulatory activity in rheumatic diseases such as rheumatoid arthritis, scleroderma and systemic lupus erythematosus. Penicillamine is capable of causing hypersensitivity reactions, some of which are accompanied by liver injury which is typically cholestatic.
Background
Penicillamine (pen" i sil' a meen) is d-isomer of dimethylcysteine, a breakdown product of penicillin, which was originally isolated from the urine of patients with liver disease receiving penicillin. It was later found to have chelating properties against copper and used in Wilson disease. Penicillamine was also found to lower levels of immune complexes, which led to its use in several rheumatic and immune disorders such as rheumatoid arthritis, scleroderma, systemic lupus erythematosus and primary biliary cirrhosis. Penicillamine was approved for use in Wilson disease in 1960 and is still widely used for that rare indication. The use of penicillamine for rheumatic disorders has decreased markedly with the availability of more modern, potent and less toxic agents. Penicillamine is available in generic forms and under the brand names of Cuprimine and Depen in capsules of 125 and 250 mg and tablets of 250 mg. The usual dose in 250 mg four times daily, increasing based upon effect and tolerance to a maximum of 2 grams daily. Lower doses were used in rheumatic diseases. Common side effects include gastrointestinal upset, metallic taste, bone marrow suppression, rash, pruritus, induction of autoimmune diseases and drug fever. Severe adverse events include aplastic anemia, agranulocytosis, thrombocytopenia, renal dysfunction, proteinuria, Goodpasture syndrome, myasthenia gravis, pemphigus and acute hypersensitivity reactions.
Hepatotoxicity
Penicillamine has been linked to a characteristic pattern of liver injury arising 1 to 6 weeks after starting therapy, with a distinctly cholestatic pattern of serum enzyme elevations (Case 1). The jaundice due to penicillamine can be severe and prolonged and result in protracted, symptomatic cholestasis, but most cases are self-limited. Immunoallergic manifestations of rash, fever and eosinophilia are common, but not invariable. Interesting, most cases of acute liver injury attributed to penicillamine were reported in patients with rheumatic diseases, only rare instances being reported in Wilson disease.
Other toxicities of penicillamine therapy such as bone marrow suppression, neutropenia and severe dermatologic features can accompany the hepatic injury. Furthermore, in instances with severe nonhepatic penicillamine toxicities, some degree of hepatic involvement such as mild-to-moderate serum enzyme elevations may occur. Long term toxicities of penicillamine include induction of autoimmune conditions (glomerulonephritis, pneumonitis, lupus-like syndrome) that may be accompanied by autoantibody formation, but autoimmune hepatitis-like syndromes have not been reported.
Likelihood score: A (well established cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury caused by penicillamine appears to be due to hypersensitivity as shown by its brief latency and the frequency of immunoallergic symptoms and signs. Susceptibility to penicillamine hypersensitivity has been linked to HLA DR3, sulphoxidation status and previous gold allergy, but these factors have not been specifically linked to hepatotoxicity from penicillamine.
Outcome and Management
The hepatotoxicity of penicillamine is typically self-limited, although recovery may be delayed. However, there have been several instances of progressive cholestasis resulting in death or need for liver transplantation arising after acute, severe cholestatic liver injury due to penicillamine. While the pathology of these cases has stressed the chronic cholestatic features and progressive fibrosis, there is little information on bile duct injury and loss. Nevertheless, these instances probably represent vanishing bile duct syndrome. Monitoring of routine liver tests is recommended before and every 6 months during therapy with penicillamine. While ursodiol and corticosteroids are often used in patients with prolonged cholestasis after hepatotoxicity from penicillamine (and other drugs), there is little evidence that they are beneficial. There may be cross reactivity between penicillamine hypersensitivity and allergic responses to penicillin, but this is not invariable. Nevertheless, caution should be employed in use of penicillins in patients with severe penicillamine hypersensitivity.
Drug Class: Antirheumatic Agents; Chelating Agents, Wilson Disease Agents
Other Drugs in the Subclass, Wilson Disease: Dimercaprol, Trientine, Zinc
CASE REPORT
Case 1. Immunoallergic hepatitis due to penicillamine.(1)
A 58 year old woman with scleroderma accompanied by arthritis, Raynaud’s phenomenon, skin tightening over the face and hands, and lung involvement was placed on penicillamine in a dose of 1.8 g daily. Three weeks later she developed fever and generalized maculopapular rash, followed two days later by dark urine and jaundice. All medications were stopped. She had no previous history of liver disease or drug allergies and did not drink alcohol. Over-the-counter medications included vitamins only. Laboratory testing showed serum bilirubin of 7.4 mg/dL, mild ALT elevations (90 U/L), and marked increases in alkaline phosphatase levels (Table). She had mild eosinophilia (5%) and leukocytosis. Over the next few days, fever and rash resolved and 3 weeks after onset, laboratory tests had returned to normal or near normal.
Key Points
| Medication: | Penicillamine (1.8 g daily) |
|---|---|
| Pattern: | Cholestatic (R=0.3) |
| Severity: | 2+ (jaundice, not hospitalized) |
| Latency: | 3 weeks |
| Recovery: | 3 weeks |
| Other medications: | Vitamin B6 |
Laboratory Values
Comment
An early report without viral serology to exclude acute viral hepatitis or modern imaging to exclude biliary obstruction, but the progression of symptoms and laboratory test abnormalities were very typical of drug induced hypersensitivity and liver injury. The latency to onset of symptoms of fever, rash and malaise was 2 to 3 weeks, with jaundice appearing soon thereafter. Immunoallergic symptoms and signs were present and the pattern of serum enzyme elevations was distinctly cholestatic, features that are typical of penicillamine induced liver injury. The features of hypersensitivity usually resolve within a week of stopping therapy, whereas the cholestasis and jaundice are slower to improve. Corticosteroids appear to improve the immunoallergic symptoms and signs promptly, but their effect on the liver injury is less clear.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Penicillamine – Generic, Cuprimine®
DRUG CLASS
Antirheumatic Agents
Chelating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Penicillamine | 52-67-5 | C5-H11-N-O2-S |
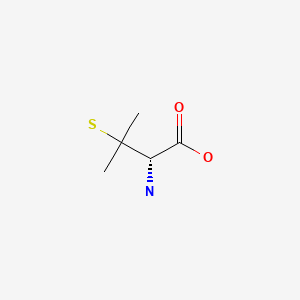
|
CITED REFERENCE
- 1.
- Rau R, Weber S, Böni A. Schweiz Med Wochenschr. 1972;102:1226–8. [Allergic-toxic liver damage due to D-penicillamine] German. [PubMed: 5055042]
ANNOTATED BIBLIOGRAPHY
References updated: 25 July 2020
- Zimmerman HJ. Drugs used in rheumatic diseases: penicillamine. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 542.(Expert review of hepatotoxicity published in 1999; mentions that there have been 18 published cases of jaundice due to penicillamine, almost all cholestatic with latency of 2-4 weeks and features of hypersensitivity).
- Byrns MC, Penning TM. Treatment of metal exposure. Environmental toxicology: carcinogens and heavy metals. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1311-5.
- Walshe JM. Penicillamine, a new oral therapy for Wilson's disease. Am J Med. 1956;21:487–95. [PubMed: 13362281](Initial studies on efficacy of oral penicillamine [β,β-dimethyl cysteine, a monothiol] in inducing cupruresis in Wilson disease and lack of effect of cysteine and methionine; no toxic reactions were observed).
- Walshe JM. Toxic reactions to penicillamine in patients with Wilson’s disease. Postgrad Med J. 1968 Suppl:6–8. [PubMed: 5706614](Summary of side effects of penicillamine representing 126 patient years of use in Wilson disease; important toxicities included nephrotic syndrome, lupus-like syndrome, thrombocytopenia, urticaria and one case of a 9 year old with urticaria, fever, malaise and jaundice, with positive rechallenge and inability to tolerate the drug and resultant death from Wilson disease).
- Sternlieb I, Scheinberg IH. Prevention of Wilson's disease in asymptomatic patients. N Engl J Med. 1968;278:352–9. [PubMed: 5635646](Among 42 asymptomatic persons who were diagnosed with Wilson disease and treated with penicillamine for up to 8 years, none developed symptomatic disease).
- Rau R, Weber S, Böni A. Schweiz Med Wochenschr. 1972;102:1226–8. [Allergic-toxic liver damage due to D-penicillamine] German. [PubMed: 5055042](58 year old with scleroderma developed fever, rash and jaundice 2-3 weeks after starting penicillamine with bilirubin 7.4 mg/dL, ALT 90 U/L, Alk P 8 times ULN, with resolution within 2 weeks of stopping: see Case 1).
- Walshe JM. Copper chelation in patients with Wilson’s disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med. 1973;42:441–52. [PubMed: 4728043](Trientine induced a cupruresis in 18 patients with Wilson disease, which was less than with penicillamine, but was substantial particularly in patients who had never been treated, suggesting that it might be an alternative for patients intolerant to penicillamine therapy).
- Siegmund H. Med Welt. 1976;27(4):172–3. [Intrahepatic cholestasis following treatment with D-penicillamine and indomethacin] German. [PubMed: 1250131](38 year old woman with rheumatoid arthritis developed jaundice 5 weeks after starting penicillamine and indomethacin [bilirubin 5.5 mg/dL, ALT 120 U/L, Alk P 10 times ULN], jaundice resolving within 4 weeks of stopping both drugs, but Alk P remained high).
- Sacher M, Thaler H. Toxic hepatitis after therapeutic doses of benorylate and D-penicillamine. Lancet. 1977;1:481–2. [PubMed: 65582](13 year old girl with rheumatoid arthritis developed jaundice 5 months after starting penicillamine with recovery in 3 months; no specifics given; benorylate is an ester of acetylsalicylic acid and acetaminophen).
- Barzilai D, Dickstein G, Enat R, Bassan H, Lichtig C, Gellei B. Case report: cholestatic jaundice caused by D-penicillamine. Ann Rheum Dis. 1978;37:98–100. [PMC free article: PMC1000201] [PubMed: 629612](56 year old man with rheumatoid arthritis developed jaundice and pruritus 4 weeks after starting penicillamine [bilirubin 15 mg/dL, ALT 203 U/L, Alk P 3 times ULN]; underwent laparotomy, with subsequent rise in bilirubin to 88 mg/dL, renal failure and death).
- McLeod BD, Kinsella TD. Cholestasis associated with D-penicillamine for rheumatoid arthritis. Can Med Assoc J. 1979;120:965–6. [PMC free article: PMC1819252] [PubMed: 436073](41 year old woman with rheumatoid arthritis developed jaundice 3 weeks after starting penicillamine [bilirubin 3.3 mg/dL, AST 117 U/L, Alk P 1132 U/L], jaundice resolving within 2 weeks and enzyme elevations within 11 weeks of stopping).
- Crickx L, Leger JM, Auquier L. Nouv Presse Med. 1979;8:212. [Granulomatous hepatitis and parotiditis induced by d-penicillamine in a case of rheumatoid arthritis] French. [PubMed: 554085](50 year old woman with rheumatoid arthritis developed cholestatic jaundice and parotiditis soon after starting penicillamine, liver and parotid biopsies demonstrating granulomas; no specifics given).
- Jaffe IA. Penicillamine in rheumatoid arthritis: clinical pharmacology and biochemical properties. Scand J Rheumatol Suppl. 1979;(28):58–64. [PubMed: 287192](Reports that penicillamine leads to improvements in stiffness and pain in rheumatoid arthritis after 8-12 weeks of therapy and can induce a variety of autoimmune conditions including pemphigus, myasthenia, glomerulonephritis, polymyositis, hemolytic anemia, lupus, Sjögren's syndrome and thrombocytopenic purpura).
- Baum J. The use of penicillamine in the treatment of rheumatoid arthritis and scleroderma. Scand J Rheumatol Suppl. 1979;(28):65–70. [PubMed: 377472](In review of literature on 1190 patients treated with penicillamine for 4-12 months, beneficial response rates averaged 62%, but significant side effects found in 37% of patients).
- Hill HFH. Penicillamine in rheumatoid arthritis: adverse effects. Scand J Rheumatol Suppl. 1979;(28):94–9. [PubMed: 156393](Review of skin adverse events and fatalities due to penicillamine, deaths largely due to bone marrow toxicity; no mention of hepatotoxicity or cholestasis).
- Wollheim FA, Lindström CG. Liver abnormalities in penicillamine treated rheumatoid arthritis. Scand J Rheumatol Suppl. 1979;(28):100–7. [PubMed: 287188](Among 99 patients followed prospectively during penicillamine therapy, 6 developed serum enzyme abnormalities without jaundice, reversible within weeks of stopping in all, not always recurring with restarting penicillamine at lower doses).
- Rosenbaum J, Katz WA, Schumacher HR. Hepatotoxicity associated with use of D-penicillamine in rheumatoid arthritis. Ann Rheum Dis. 1980;39:152. [PMC free article: PMC1000500] [PubMed: 7387219](Two patients with rheumatoid arthritis with penicillamine hepatotoxicity; 43 year old woman developed enzyme elevations [normal bilirubin, AST 440 U/L, Alk P ~2 times ULN] without symptoms 2 months after starting penicillamine, resolving rapidly on stopping and recurring with rechallenge; 60 year old man developed jaundice 2 weeks after starting penicillamine [bilirubin 2.2 mg/dL, AST 128 U/L, Alk P 8 times ULN], resolving slowly over next 9 months).
- Seibold JR, Lynch CJ, Medsger TA. Cholestasis associated with D-penicillamine therapy: case report and review of the literature. Arthritis Rheum. 1981;24:554–6. [PubMed: 7213433](26 year old woman with lupus developed fever and rash after 10 days of penicillamine which improved on stopping, but recurred after a single tablet of penicillamine with fever, rash, arthritis followed by jaundice [bilirubin 7.9 mg/dL, ALT 315 U/L, Alk P 358 U/L], resolving within 7 weeks).
- Jensen OH. Ugeskr Laeger. 1981;143:3471–2. [Penicillamine induced liver involvement] Danish. [PubMed: 7336507](64 year old man with rheumatoid arthritis developed jaundice after a year of penicillamine therapy and had sudden worsening when it was inadvertently restarted with fever and jaundice [bilirubin ~3.0 mg/dL, Alk P and AST 3 times ULN], symptoms resolving rapidly on stopping and liver tests returning to normal within 3 months).
- Multz CV. Cholestatic hepatitis caused by penicillamine. JAMA. 1981;246:674–5. [PubMed: 7253120](53 year old woman with rheumatoid arthritis developed jaundice 4 weeks after starting penicillamine [bilirubin 6.4 mg/dL, AST 390 U/L, Alk P 820 U/L], jaundice resolving in 2 weeks; remission in arthritis during period of jaundice).
- Job-Deslandre C, Delrieu F, Rondier J, Guedri M, Delbarre F. Hepatitie cholestatique et anticorps anti-DNA induits par la d-penicillamine. Nouv Presse Med. 1982;11:2356–7. [PubMed: 6981100](33 year old with rheumatoid arthritis treated with penicillamine for 4 years developed jaundice and lupus-like syndrome [bilirubin not given, ALT 61 U/L, Alk P 3.6 times ULN, ANA 1:500], liver abnormalities resolving within 2 months, but ANA remaining positive).
- Grauer JL, Fonteille J, Zarski JP, Gintz B, Phelip X, Cabanel G. Presse Med. 1983;12:1997. [Erythema nodosum and cholestatic hepatitis during treatment with D-penicillamine] French. [PubMed: 6225106](62 year old with rheumatoid arthritis developed fever 20 days after starting penicillamine, resolving on stopping and reappearing 2 months after restarting along with rash, but no jaundice [bilirubin not given, AST 1.5 times, Alk P 4.5 times ULN], resolving within 2 weeks of stopping again).
- Emery P, Panayi GS, Huston G, Welsh KI, Mitchell SC, Shah RR, Idle JR, et al. D-penicillamine induced toxicity in rheumatoid arthritis : the role of sulphoxidation status and HLA-DR3. J Rheumatol. 1984;11:626–32. [PubMed: 6334741](Among 66 patients with rheumatoid arthritis treated with penicillamine, adverse events [largely renal, bone marrow and skin toxicities] were more common in those with low sulphoxidation indices [60%] than in those with normal values [17%], and with HLA DR3 [60% vs 29%], without interaction of the two predictive factors).
- Gefel D, Hrats N, Lijovetsky G, Eliakim M. Cholestatic jaundice associated with d-penicillamine therapy. Scand J Rheumatol. 1985;14:303–6. [PubMed: 4048879](35 year old man with cystinuria developed fever and rash 16 days after starting penicillamine followed by jaundice [peak bilirubin 14.9 mg/dL, ALT 149 U/L, Alk P 553 U/L], biopsy showing intrahepatic cholestasis; prednisone was started when bilirubin worsened; jaundice resolved within 3 months and abnormal Alk P within 9 months of stopping).
- Devogelaer JP, Huaux JP, Coche E, Rahier J, Nagant de Deuxchaisnes C. A case of cholestatic hepatitis associated with D-penicillamine therapy for rheumatoid arthritis. Int J Clin Pharmacol Res. 1985;5:35–8. [PubMed: 3997313](72 year old man with rheumatoid arthritis developed pruritus and jaundice 1 month after starting penicillamine [bilirubin 16.2 mg/dL, ALT 227 U/L, Alk P 290 U/L], jaundice resolving within 3 weeks and serum enzymes within 6 weeks of stopping).
- Abadia R, Jammet P, Christoforov B, Cremer GA. Ann Med Interne (Paris). 1985;136:590–600. [Hepatotoxicity of drugs commonly used in rheumatology] [PubMed: 2868682](Review of hepatotoxicity of rheumatologic agents; mentions 16 cases of penicillamine induced liver injury, typically with immunoallergic features and cholestatic pattern with resolution in 2-4 weeks, recurrence after reexposure in 5 instances; one fatality).
- Kumar A, Bhat A, Gupta DK, Goel A, Malaviya AN. D-penicillamine-induced acute hypersensitivity pneumonitis and cholestatic hepatitis in a patient with rheumatoid arthritis. Clin Exp Rheumatol. 1985;3:337–9. [PubMed: 4085166](45 year old woman with rheumatoid arthritis developed rash, fever and hypersensitivity pneumonitis 10 days after starting penicillamine, responding to prednisone therapy but with subsequent jaundice [peak bilirubin 30 mg/dL, ALT 300 U/L, Alk P 5 times ULN], resolving within 4 weeks of stopping; recurrence of dyspnea and jaundice after unintentional rechallenge).
- Choudhuri G, Tandon RK. D-penicillamine induced cholestatic jaundice. J Assoc Physicians India. 1986;34:299–300. [PubMed: 3759835](45 year old woman with rheumatoid arthritis developed rash, fever and dyspnea within 2 weeks of starting penicillamine [initial bilirubin 7.0 mg/dL, ALT 200 U/L, Alk P 5 times ULN]; allergic alveolitis responding rapidly to prednisone, but bilirubin rising to 30 mg/dL; positive rechallenge; appears to be same patient as described by Kumar [1985]).
- Roux H, Bonnefoy-Cudraz M, Antipoff GM. Rev Rhum Mal Osteoartic. 1986;53:21–3. [Liver complications caused by D-penicillamine. Apropos of a case] French. [PubMed: 3704507](60 year old woman with rheumatoid arthritis developed rash and pruritus followed by jaundice 6 weeks after starting penicillamine [bilirubin 8.2 mg/dL, ALT 61 U/L, Alk P 2158 U/L, 14% eosinophilia], resolving slowly upon stopping).
- Scheinberg IH, Jaffe ME, Sternlieb I. The use of trientine in preventing the effects of interrupting penicillamine therapy in Wilson's disease. N Engl J Med. 1987;317:209–13. [PubMed: 3600712]
- Cooperative Systemic Studies of Rheumatic Disease Group. Toxicity of longterm low dose D-penicillamine therapy in rheumatoid arthritis. J Rheumatol. 1987;14:67–73. [PubMed: 2952797](Among 148 patients with rheumatoid arthritis continued on long term penicillamine, side effects included rash, gastrointestinal upset, proteinuria, bone marrow suppression, myasthenia, myositis, drug fever and pemphigus; no late hepatotoxicity).
- Moens HJ, Ament BJ, Feltkamp BW, van der Korst JK. Longterm followup of treatment with D-penicillamine for rheumatoid arthritis: effectivity and toxicity in relation to HLA antigens. J Rheumatol. 1987;14:1115–9. [PubMed: 3437418](HLA testing done on 86 patients with rheumatoid arthritis started on penicillamine including 22 on long term [5-7 years] therapy; HLA-DR4 was associated with thrombocytopenia [94% vs 67%] and HLA-B8/DR3 with proteinuria [60% vs 9%], but no association with effectiveness; no cases of hepatotoxicity).
- Berbis P, Fabre JF, Privat Y. Ann Dermatol Venereol. 1987;114:377–9. [Cholestatic hepatitis: a rare complication of the treatment with D. penicillamine] French. [PubMed: 3605968](22 year old woman with scleroderma developed fever, rash, and weakness followed by jaundice 10 days after starting penicillamine [bilirubin 1.5 mg/dL, ALT 381 U/L, Alk P 409 U/L with mild eosinophilia], resolving within 3 weeks of stopping).
- Fishel B, Tishler M, Caspi D, Yaron M. Fatal aplastic anaemia and liver toxicity caused by d-penicillamine treatment of rheumatoid arthritis. Ann Rheum Dis. 1989;48:609–10. [PMC free article: PMC1003826] [PubMed: 2774703](65 year old woman with rheumatoid arthritis developed thrombocytopenia 3 months after starting penicillamine [platelet count 10,000/µL, white cell count 3000/µL, hemoglobin 6.7 gm/dL] and subsequently developed jaundice [bilirubin 10.0 mg/dL, AST 80 U/L, Alk P 120 U/L], dying 17 days later with bone marrow, renal and hepatic failure).
- Jacobs JW, Van der Weide FR, Kruijsen MW. Fatal cholestatic hepatitis caused by D-penicillamine. Br J Rheumatol. 1994;33:770–3. [PubMed: 8055207](37 year old woman with rheumatoid arthritis developed fever, granulocytopenia and jaundice 10 days after starting penicillamine [without rash or eosinophilia], with intrahepatic cholestasis on liver biopsy and subsequent persistent jaundice and cholestasis and death from sepsis 14 months later).
- Barthel HR, Hiepe F. Dtsch Med Wochenschr. 1995;120:1253–7. [Lupus erythematosus and liver diseases] German. [PubMed: 7671785](Review of liver abnormalities in lupus erythematosus; penicillamine can induce a lupus-like syndrome which resolves when the drug is stopped, although ANA positivity may persist).
- Harders H, Cohnen E. Preparation of and clinical experiences with trien for the treatment of Wilson’s disease in absolute intolerance of D-penicillamine. Proc R Soc Med. 1977;70 Suppl 3:10–2. [PMC free article: PMC1543601] [PubMed: 122652](28 year old woman who was intolerant to penicillamine was successfully treated with trientine prepared by an improved method, with prompt cupruresis and improvement in neurologic symptoms).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to penicillamine).
- Roberts EA, Schilsky ML. AASLD. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–111. [PubMed: 18506894](Thorough review of the cause, natural history, diagnosis and treatment of Wilson disease with specific recommendations for use of penicillamine, trientine and zinc).
- Walshe JM. The conquest of Wilson's disease. Brain. 2009;132(Pt 8):2289–95. [PubMed: 19596747](History of the initial description of Wilson disease, its link to copper accumulation, and therapies several of which were developed by the author).
- Weiss KH, Stremmel W. Evolving perspectives in Wilson disease: diagnosis, treatment and monitoring. Curr Gastroenterol Rep. 2012;14(1):1–7. [PubMed: 22083169](Review of the diagnosis and management of Wilson disease, including the role of genetic testing and the choice of medical therapies).
- Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, Merle U, et al. EUROWILSON Consortium. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028–35.e1. [PubMed: 23542331](Retrospective analysis of 380 patients with Wilson disease from referral centers in Germany and Austria, including 141 who were treated with trientine and 326 with penicillamine, found similar rates of improvement in liver disease but higher rate of improvement in neurologic features with penicillamine, along with higher rates of adverse events leading to discontinuation [29% vs 7%], although there were no therapy related deaths; reasons for discontinuation in the penicillamine group included arthralgias, gastrointestinal upset, myalgias, leukopenia, rash, lupus erythematosus and increase in ANA titers; no mention of ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which was attributed to penicillamine or other agents used to treat Wilson disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to penicillamine in a woman with scleroderma who developed fatigue, jaundice and rash 3 weeks after starting penicillamine [bilirubin 5.6 rising to 10.5 mg/dL, ALT 308 U/L, Alk P 377 U/L], resolving slowly and with persistent, mild elevations in ALT and Alk P during long term follow up).
- Pfeiffenberger J, Beinhardt S, Gotthardt DN, Haag N, Freissmuth C, Reuner U, Gauss A, et al. Pregnancy in Wilson's disease: management and outcome. Hepatology. 2018;67:1261–9. [PubMed: 28859232](Among 282 pregnancies in 136 patients with Wilson disease analyzed in a retrospective, multicenter study, spontaneous abortion was less common among patients being treated with penicillamine [17%] or zinc [10%] than untreated patients [41%], which was proportionally greater than with trientine [28%] and similar to that in patients who discontinued therapy during pregnancy [36%]; worsening of liver disease arose in some patients on treatment but was self-limiting and resolved after delivery; birth defects were found in 7 of 209 [3%] newborns overall and in 4 of 98 women taking penicillamine).
- Roberts EA. Update on the diagnosis and management of Wilson disease. Curr Gastroenterol Rep. 2018;20:56. [PubMed: 30397835](Review of recent advances in the understanding of the pathogenesis, clinical features, diagnosis, and treatment of Wilson disease).
- Appenzeller-Herzog C, Mathes T, Heeres MLS, Weiss KH, Houwen RHJ, Ewald H. Comparative effectiveness of common therapies for Wilson disease: A systematic review and meta-analysis of controlled studies. Liver Int. 2019;39:2136–52. [PubMed: 31206982](Systematic review of literature on therapies of Wilson disease found no differences in outcomes with penicillamine vs zinc, but higher rates of adverse events and discontinuations with penicillamine; no mention of hepatotoxicity or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Penicillamine revisited: historic overview and review of the clinical uses and cutaneous adverse effects.[Am J Clin Dermatol. 2013]Review Penicillamine revisited: historic overview and review of the clinical uses and cutaneous adverse effects.Ishak R, Abbas O. Am J Clin Dermatol. 2013 Jun; 14(3):223-33.
- Review Wilson Disease Agents.[LiverTox: Clinical and Researc...]Review Wilson Disease Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Systemic lupus erythematosus during penicillamine therapy for rheumatoid arthritis.[Ann Intern Med. 1982]Systemic lupus erythematosus during penicillamine therapy for rheumatoid arthritis.Chalmers A, Thompson D, Stein HE, Reid G, Patterson AC. Ann Intern Med. 1982 Nov; 97(5):659-63.
- Penicillamine-induced rapidly progressive glomerulonephritis in patients with progressive systemic sclerosis: successful treatment of two patients and a review of the literature.[Am J Kidney Dis. 1986]Penicillamine-induced rapidly progressive glomerulonephritis in patients with progressive systemic sclerosis: successful treatment of two patients and a review of the literature.Ntoso KA, Tomaszewski JE, Jimenez SA, Neilson EG. Am J Kidney Dis. 1986 Sep; 8(3):159-63.
- Penicillamine-induced dermatomyositis. A case history.[Scand J Rheumatol. 1983]Penicillamine-induced dermatomyositis. A case history.Lund HI, Nielsen M. Scand J Rheumatol. 1983; 12(4):350-2.
- Penicillamine - LiverToxPenicillamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...