NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Oxacillin is a parenteral, second generation penicillin antibiotic that is used to treat moderate-to-severe, penicillinase-resistant staphylococcal infections. Oxacillin has been linked to rare instances of clinically apparent, idiosyncratic liver injury, but it more commonly causes transient elevations in serum aminotransferases without jaundice.
Background
Oxacillin (ox" a sil' in) is a second generation penicillin that is highly resistant to inactivation by penicillinases and is used to treat moderate-to-severe bacterial infections caused by penicillinase-producing bacteria, particularly staphylococcal infections. Oxacillin was approved for use in the United States in 1989 and is still in common use. Oxacillin is indicated for moderate-to-severe bacterial infections caused by sensitive agents including acute or chronic osteomyelitis and valvular endocarditis. Oxacillin is available in both oral and parenteral preparations in several generic formulations. Parenteral oxacillin is recommended in doses of 250 or 500 mg intramuscularly or intravenously every 4 to 6 hours daily for up to 1 to 8 weeks depending upon the type and severity of infection. Oral oxacillin is recommended in doses of 500 mg every 4 to 6 hours, but is now rarely used. Common side effects include fatigue, anxiety, dizziness, diarrhea, nausea, fever and hypersensitivity reactions. Rare but potentially severe adverse events include anaphylaxis, Clostridium difficile diarrhea and neutropenia.
Hepatotoxicity
Oxacillin has been linked to two forms of hepatotoxicity, first an acute and transient elevation in serum aminotransferase levels occurring with high doses of intravenous therapy; and second, a more prolonged, usually cholestatic, idiosyncratic liver injury that is similar to the hepatotoxicity of other second-generation penicillins such as dicloxacillin, flucloxacillin, and nafcillin.
High doses of intravenous oxacillin are commonly accompanied by elevations in serum ALT in the range of 2 to 20 times the upper limit of normal arising after 1 to 3 weeks of therapy. Alkaline phosphatase levels are only minimally elevated. Fever and nonspecific symptoms of abdominal pain and nausea can occur, but are often absent. Eosinophilia is present in some patients, but rash and arthralgias are uncommon. Serum aminotransferase levels rapidly fall into the normal range (in 1 to 2 weeks) with discontinuation of oxacillin or switch to lower doses, particularly in oral formulations. Jaundice does not occur. There appears to be no cross reactivity of this response with the natural penicillins, clindamycin or even nafcillin. Intravenous carbenicillin can cause a similar syndrome. This hepatotoxicity may be more common in HIV-positive than noninfected individuals.
In addition to the common syndrome of asymptomatic serum aminotransferase elevations during high dose intravenous therapy, oxacillin can also but rarely lead to a more prolonged usually cholestatic hepatitis that appears 1 to 6 weeks after starting therapy and may persist for weeks to months. This form of idiosyncratic liver injury is similar to that described with dicloxacillin and other second generation penicillins. Immunoallergic features of rash, fever and eosinophilia can occur, but are not prominent. Autoantibodies are not found. The liver injury can be prolonged, but generally resolves within 1 to 2 months of onset. Liver biopsy generally shows a cholestatic hepatitis with mixed inflammatory infiltrates.
Likelihood score: B (likely rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of ALT elevations during high dose oxacillin therapy is not known, but may be due to a direct but mild injury to the liver. Fever is common, and eosinophilia and rash can occur. Liver biopsy during these episodes generally shows mild, focal cell injury. The idiosyncratic reaction to oxacillin (and other related penicillins) is rarely accompanied by signs of hypersensitivity or allergy, but has some characteristics that suggest such a mechanism, such as the rapid reappearance of injury with reexposure, and an association with a history of penicillin allergy. Too few cases of oxacillin cholestatic injury have been reported to comment on possible HLA associations, such as the link to HLA-B*5701 which has been made to flucloxacillin.
Outcome and Management
The serum aminotransferase elevations that appear during high dose intravenous therapy with oxacillin are usually benign, asymptomatic and resolve rapidly with stopping therapy or switching to other forms of penicillinase-resistant penicillins. The cholestatic hepatitis that occurs very rarely can be symptomatic and prolonged, but has not been linked to acute, liver failure, chronic or permanent injury, or vanishing bile duct syndrome. Prednisone has been used to treat the cholestatic liver injury, but its effects are unclear while its side effects can be serious. Patients should be told to avoid reexposure to the penicillinase-resistant penicillins, including nafcillin and dicloxacillin.
Drug Class: Penicillin (Penicillinase-Resistant)
CASE REPORT
Case 1. Elevated aminotransferase levels during intravenous oxacillin therapy.(1)
A 52 year old man with multiple medical problems was treated with intravenous oxacillin for suspected osteomyelitis and was found to have elevations in ALT (104 U/L) and AST (82 U/L), with normal alkaline phosphatase and bilirubin levels when tested 7 days later. He had no symptoms that could be attributed to liver injury. Oxacillin was continued, but ALT levels continued to rise. Oxacillin was stopped after 6 weeks. Serum aminotransferase levels promptly fell and were near normal when he was discharged 2 weeks later. This patient also had multiple other medical problems including hypertension, diabetes, chronic hepatitis C, gastrointestinal reflux, chronic obstructive lung disease, depression and chronic radiculopathy for which he took many medications, none of which had been changed recently.
Key Points
| Medication: | Oxacillin |
|---|---|
| Pattern: | Hepatocellular (ALT elevations only) |
| Severity: | 1+ (aminotransferase elevations without jaundice) |
| Latency: | One week |
| Recovery: | Almost complete within 2 weeks of discontinuation |
| Other medications: | Amlodipine, metoprolol, insulin, lansoprazole, levothyroxine, gabapentin, trimethoprim-sulfamethoxazole, diazepam, cyclobenzaprine, docusate, senna, iron, naproxen |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| - 1 week | 16 | 54 | 0.7 | Admitted with osteomyelitis | |
| 0 | Oxacillin started: 2 grams iv every 6 hours | ||||
| 7 days | 104 | 79 | 0.6 | ||
| 38 days | 108 | 92 | 0.7 | ||
| 40 days | 306 | 98 | 0.7 | ||
| 41 days | Oxacillin stopped | ||||
| 43 days | 2 days | 318 | 99 | 0.7 | |
| 46 days | 5 days | 85 | |||
| 49 days | 9 days | 57 | 85 | 0.6 | |
| 52 days | 12 days | 59 | 80 | 0.6 | |
| Normal Values | <40 | <115 | <1.2 | ||
Comment
Oxacillin is well known to cause serum ALT elevations when given in high doses intravenously. The elevations are usually mild to moderate (<20 fold elevated) and appear after 4 to 20 days. Most patients are asymptomatic, but complaints of abdominal discomfort or fever may arise. Alkaline phosphatase and bilirubin levels remain normal. Patients usually tolerate other intravenous antibiotics (including nafcillin) without recurrence and the injury is self-limited, not leading to jaundice or severe liver disease even if oxacillin is continued. The presence of an underlying chronic hepatitis C complicates the interpretation of this case and many of the cases in the literature. However, serum ALT levels were normal before oxacillin was started and returned towards baseline once it was stopped, making it likely that the changes were due to the drug.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Oxacillin – Generic
DRUG CLASS
Penicillin (Penicillinase-Resistant)
Product labeling at DailyMed, National Library of Medicine, NIH
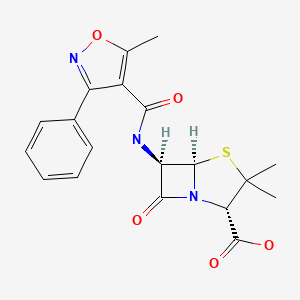
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Oxacillin | 66-79-5 | C19-H19-N3-O5-S |
|
CITED REFERENCES
- 1.
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056]
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Synthetic penicillins. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 596-8.(Expert review of penicillins and liver injury published in 1999).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics mentions that liver injury from the penicillins is very rare, and is usually cholestatic for the oxypenicillins such as dicloxacillin and cloxacillin).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Freedman MA. Oxacillin—apparent hematologic and hepatic toxicity. Rocky Mt Med J. 1965;62:34–6. [PubMed: 14224480](33 year old woman developed AST elevations to ~220 U/L, atypical lymphocytosis and eosinophilia after 2.5 months of high dose oral oxacillin, complicated by bone marrow suppression and breakthrough in chronic osteomyelitis).
- Pas AT, Quinn EL. Cholestatic hepatitis following the administration of sodium oxacillin. JAMA. 1965;191:674–5. [PubMed: 14242432](65 year old woman developed itching and jaundice the week after stopping a 4 week course of oral oxacillin [bilirubin 2.2 mg/dL, Alk P ~twice elevated, AST 76 U/L], resolving within 8 weeks).
- Dismukes WE. Oxacillin-induced hepatic dysfunction. JAMA. 1973;226:861–3. [PubMed: 4800332](3 cases of ALT elevations, fever and nausea arising 11-24 days after starting high dose intravenous oxacillin therapy, with rapid reversal on stopping).
- Klein I, Tobias H. Oxacillin-associated hepatitis. Am J Gastroenterol. 1976;65:546. [PubMed: 961686](23 year old male injection drug user developed ALT elevations [~275 U/L] without symptoms or change in bilirubin or Alk P after 10 days of intravenous oxacillin [16 g/day), with rapid resolution [<1 week] on stopping).
- Olans RN, Weiner LB. Reversible oxacillin hepatotoxicity. J Pediatr. 1976;89:835–8. [PubMed: 978335](8 instances of ALT elevations [peak values 31-1359 U/L] without jaundice [2 had eosinophilia] after 8-25 days of intravenous oxacillin in children or young adults, with rapid resolution; no recurrence when switched to nafcillin).
- Bruckstein AH, Attia AA. Oxacillin hepatitis. Two patients with liver biopsy, and review of the literature. Am J Med. 1978;64:519–22. [PubMed: 637061](Two injection drug users treated with high dose oxacillin [12-16 g/day] for endocarditis developed increased AST levels [10-40 times ULN], with mild symptoms but no change in Alk P or bilirubin and nonspecific biopsy findings, arising after 2-3 weeks and resolving rapidly with stopping).
- Goldstein LI, Granoff M, Waisman J. Hepatic injury due to oxacillin administration. Am J Gastroenterol. 1978;70:171–4. [PubMed: 717369](21 year old male injection drug used developed aminotransferase elevations [ALT 806 U/L, AST 388 U/L, 15% eosinophils] 20 days after starting intravenous oxacillin [12 g/day], with biopsy showing hepatitis, rapid recovery).
- Onorato IM, Axelrod JL. Hepatitis from intravenous high-dose oxacillin therapy: findings in an adult inpatient population. Ann Intern Med. 1978;89:497–500. [PubMed: 697229](Review of experience with high dose oxacillin revealed 8 cases of liver injury, rising 3-19 days after start of therapy with fever and mild GI symptoms, ALT 2 to 15 times ULN, Alk P minimally increased, and no jaundice; resolving in 1 week; compared to control group, a history of penicillin allergy was given in 3 cases, but not controls).
- D'Angelo LJ. Oxacillin and hepatotoxicity. Ann Intern Med. 1979;90:442. [PubMed: 426429](Letter commenting on article by Onorato & Axelrod, without new data).
- Taylor C, Corrigan K, Steen S, Craig C. Oxacillin and hepatitis. Ann Intern Med. 1979;90:857–8. [PubMed: 434706](Letter in response to Onorato & Axelrod describing 24 year old man who developed ALT elevations [226 U/], with rash and eosinophilia after 27 days of intravenous oxacillin therapy [8 g/day], rapidly resolving upon switching to nafcillin).
- Pollock AA, Berger SA, Simberkoff MS, Rahal JJ Jr. Hepatitis associated with high-dose oxacillin therapy. Arch Intern Med. 1978;138:915–7. [PubMed: 646563](Prospective study of liver tests in 41 patients during intravenous oxacillin therapy; 15 had ALT elevations, but only 5 were attributed to oxacillin, 12-23 g/day arising 5-24 days after starting, peak ALT values 5-20 times ULN, mild Alk P elevation in 4, but normal bilirubin in all, and resolution in 4-16 days of stopping; no eosinophilia, fever or symptoms).
- Halloran TJ, Clague MD. Hepatitis associated with high-dose oxacillin therapy. Arch Intern Med. 1979;139:376–7. [PubMed: 426588](Letter in response to Pollock [1978] describing 16 year old girl with no history of injection drug use who developed AST elevations [peak 140 U/L], starting after 9 days of intravenous oxacillin, rapid resolution upon stopping).
- Nahata MC, DeBolt SL, Powell DA. Adverse effects of methicillin, nafcillin and oxacillin in pediatric patients. Dev Pharmacol Ther. 1982;4:117–23. [PubMed: 7172968](Prospective study of children treated with intravenous methicillin [28], nafcillin [32] or oxacillin [8]; minimal ALT elevations occurred in 1 on nafcillin and 1 on oxacillin; no jaundice).
- Miller WI, Souney PF, Chang JT. Hepatic dysfunction following nafcillin and cephalothin therapy in a patient with a history of oxacillin hepatitis. Clin Pharm. 1983;2:465–8. [PubMed: 6627877](Mild ALT elevations after 13 days of high dose oxacillin [18 g/day], similar increase after nafcillin and again with cephalosporin in a male injection drug user with probable chronic hepatitis C, which might have accounted for ALT fluctuations).
- Tauris P, Jørgensen NF, Petersen CM, Albertsen K. Prolonged severe cholestasis induced by oxacillin derivatives. A report on two cases. Acta Med Scand. 1985;217:567–9. [PubMed: 4025011](2 case reports: 65 year old man with osteomyelitis developed jaundice and pruritus 6 weeks after starting flucloxacillin [bilirubin ~10 mg/dL, AST 1.5 times ULN, Alk P 3 times ULN], biopsy showing cholestasis, resolving within 8 weeks of stopping; 21 year old man with osteomyelitis developed jaundice 9 weeks after starting dicloxacillin [bilirubin ~4 mg/dL, AST 119 U/L, Alk P 814 U/L], resolving within 6 weeks of stopping).
- Saliba B, Herbert PN. Oxacillin hepatotoxicity in HIV-infected patients. Ann Intern Med. 1994;120:1048. [PubMed: 8185140](Retrospective review found that 81% [9/11] of HIV-positive, but only 4.5% [3/66] of HIV-negative patients receiving iv oxacillin for more than 10 days developed ALT or AST >3 fold elevated).
- Al-Homaidhi H, Abdel-Haq NM, El-Baba M, Asmar BI. Severe hepatitis associated with oxacillin therapy. South Med J. 2002;95:650–2. [PubMed: 12081223](6 year old girl developed fever, abdominal pain and marked ALT elevation [peak 2,257 U/L] with normal Alk P and bilirubin [0.3 mg/dL] after 14 days of intravenous oxacillin, resolving within 3 weeks of stopping).
- Trevenzoli M, Cattelan AM, Mencarelli R, Meneghetti F. Severe hepatitis associated with oxacillin therapy. South Med J. 2003;96:324–5. [PubMed: 12659378](Letter in response to Al-Homaidhi [2002] describing 33 year old who developed liver injury [bilirubin 1.8 mg/dL, ALT 2440 U/L, Alk P 103 U/L] weeks after receiving a 4 day course of amoxicillin/clavulanate [not oxacillin as suggested by the title]).
- Ibáñez L, Pérez E, Vidal X, Laporte JR. Grup d'Estudi Multicènteric d'Hepatotoxicitat Aguda de Barcelona (GEMHAB). Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol. 2002;37:592–600. [PubMed: 12399224](Survey of 107 cases of acute serious liver disease, not due to viruses, found no instances of drug induced liver injury due to penicillinase-resistant penicillins).
- Maraqa NF, Gomez MM, Rathore MH, Alvarez AM. Higher occurrence of hepatotoxicity and rash in patients treated with oxacillin, compared with those treated with nafcillin and other commonly used antimicrobials. Clin Infect Dis. 2002;34:50–4. [PubMed: 11731945](Retrospective analysis of laboratory tests from 222 children receiving outpatient parenteral oxacillin, nafcillin, clindamycin or other antibiotics found 12 cases of anicteric and self-limited hepatotoxicity, 9 [22%] from oxacillin, all hepatocellular with normal bilirubin, onset in 6-43 days, resolution in 1-3 weeks; none to nafcillin; 1 clindamycin, 1 ceftriaxone and 1 ampicillin/sulbactam/gentamicin).
- Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, Alfirevic A, et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–39. [PubMed: 22987284](T cells from persons with HLA-B*57:01 were activated when exposed to dendritic cells presenting flucloxacillin bound to albumin, and were similarly activated by oxacillin, cloxacillin and dicloxacillin).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Analysis of all fatal adverse drug event reports of liver injury in Sweden between 1966 and 2002, found 103 cases; most common causes were halothane [n=16], acetaminophen [14], flucloxacillin [9], and TMP-SMZ [6]).
- Hussaini SH, O'Brien CS, Despott EJ, Dalton HR. Antibiotic therapy: a major cause of drug-induced jaundice in southwest England. Eur J Gastroenterol Hepatol. 2007;19:15–20. [PubMed: 17206072](Review of causes of non-obstructive jaundice in 347 patients presenting between 1998 and 2004 at a single UK center, 28 were thought to be drug induced liver injury [8.1%] and antibiotics were the most common cause, 32% amoxicillin/clavulanate, 25% flucloxacillin, 18% other).
- Lee CY, Chen PY, Huang FL, Chi CS. Reversible oxacillin-associated hepatitis in a 9-month old boy. J Paediatr Child Health. 2008;44:146–8. [PubMed: 18307421](9 month old boy given intravenous oxacillin for septic arthritis for 8 days when he developed lethargy and marked elevations in ALT [1568 U/L], with normal Alk P and bilirubin and resolving within 10 days of switching to teicoplanin).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, antimicrobials accounted for 45% of cases with 23 single agent cases due to amoxicillin/clavulanate, 13 nitrofurantoin, 10 fluoroquinolones, 9 macrolides, 9 sulfonamides, 5 cephalosporins, 3 oxacillin, 2 doxycycline, 2 amoxicillin, and one each for gentamicin, imipenem, and clindamycin, but none from dicloxacillin or nafcillin).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; leading causes were antituberculosis agents [58%], anticonvulsants [11%] and NSAIDs [2%]; specific antibiotic agents included sulfamethoxazole/ trimethoprim [2%] and amoxicillin-clavulanate [1%]).
- Ferrajolo C, Capuano A, Verhamme KMC, Schuemie M, Rossi F, Stricker BH, Sturkenboom CJM. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse drug reports in children in a worldwide pharmacovigilance database, 6595 [1%] were for hepatic injury and antibacterials accounted for 11%, those with the highest adjusted odds ratios being aztreonam, erythromycin, ceftriaxone and minocycline; no mention of penicillins).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 66 due to antimicrobial agents, but none were attributed to a penicillinase-resistant penicillin).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: pathomechanisms and clinical data. Infection. 2010;38:3–11. [PubMed: 20107858](Review of hepatotoxicity of antibiotics, mentions that cholestatic hepatitis has been reported with many beta-lactams and beta-lactamases inhibitors).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin/clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 persons with suspected drug induced liver injury seen over at 12 month period at a referral hospital in Tasmania, 11 were attributed to antibiotics including 4 to flucloxacillin, 2 amoxicillin with clavulanate, 2 amoxicillin, and 1 each to rifampin, moxifloxacin and ciprofloxacin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation and Outcomes in Patients with Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 36 [37%] due to antibiotics and specifically dicloxacillin in 1 of 22,320 patients treated and cloxacillin in 1 of 3659 patients treated).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, but none to penicillinase resistant penicillins such as oxacillin or dicloxacillin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 cases [36%] were attributed to antibiotics 3 of which were due to oxacillin, all being self-limited episodes of aminotransferase elevations without jaundice; no instances of dicloxacillin or nafcillin associated liver injury).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–1716.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome-wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nafcillin.[LiverTox: Clinical and Researc...]Review Nafcillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Reversible oxacillin hepatotoxicity.[J Pediatr. 1976]Reversible oxacillin hepatotoxicity.Olans RN, Weiner LB. J Pediatr. 1976 Nov; 89(5):835-8.
- Review Dicloxacillin.[LiverTox: Clinical and Researc...]Review Dicloxacillin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- THE ROLE OF PENICILLINASE IN STAPHYLOCOCCAL INFECTIONS.[Br J Exp Pathol. 1964]THE ROLE OF PENICILLINASE IN STAPHYLOCOCCAL INFECTIONS.SHILO M, CITRI N. Br J Exp Pathol. 1964 Apr; 45(2):192-7.
- [METHICILLIN- AND OXACILLIN-RESISTANT STAPHYLOCOCCI].[Munch Med Wochenschr. 1964][METHICILLIN- AND OXACILLIN-RESISTANT STAPHYLOCOCCI].STILLE W, HIRSCH HA. Munch Med Wochenschr. 1964 Aug 28; 106:1528-32.
- Oxacillin - LiverToxOxacillin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...