NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ofloxacin is a second generation fluoroquinolone that was previously used widely for therapy of mild-to-moderate bacterial infections, but which has been replaced by more potent and less toxic fluoroquinolones and is now used largely topically as eye and ear drops. Ofloxacin has been linked to rare instances of acute hepatocellular injury.
Background
Ofloxacin (oh flox' a sin) is an oral, second generation fluoroquinolone that was previously widely used to treat mild-to-moderate urinary and respiratory tract infections caused by susceptible organisms. Ofloxacin is a semisynthetic antibiotic and a racemic mixture; its l-enantiomer is available as levofloxacin which continues to be a widely used antibiotic. Like other fluoroquinolones, ofloxacin is active against a wide range of aerobic gram-positive and gram-negative organisms and is believed to act by inhibition of bacterial DNA gyrase and topoisomerase IV that are required for synthesis of bacterial mRNAs (transcription) and DNA replication. In contrast, DNA gyrases are not present in human [and other eukarotic] cells and the equivalent topoisomerases are not sensitive to fluoroquinolone inhibition. Ofloxacin was approved for use in the United States in 1990, but was discontinued by its initial sponsor in 2009, partially because of the frequency of adverse side effects. Nevertheless, ofloxacin remains available several in generic forms as 200, 300 and 400 mg tablets. Current indications are acute bronchitis, community acquired pneumonia, skin, infections, cystitis, pelvic inflammatory disease, prostatitis, urethritis and gonorrhea. Typical doses are 200 to 400 mg every 12 hours for 3 to 10 days, but longer courses are sometimes used for complicated or recurrent infections. Common side effects include gastrointestinal upset, headaches, skin rash and allergic reactions. Less common but more severe side effects of fluoroquinolones include prolongation of the QT interval, seizures, hallucinations, tendon rupture, hypersensitivity reactions and photosensitivity.
Hepatotoxicity
Mild elevations in ALT and alkaline phosphatase levels occur in 1 to 2% of patients on ofloxacin. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. Ofloxacin has also been linked to rare but occasionally severe and even fatal cases of acute liver injury. The time to onset is typically short (2 days to 2 weeks) and the presentation is often abrupt with nausea, fatigue, abdominal pain and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic, cases with the shorter times to onset usually being more hepatocellular with markedly elevated ALT levels, and occasionally with rapid worsening of prothrombin time and signs of hepatic failure. The onset of illness may occur a few days after the medication is stopped. Cases with a cholestatic pattern of enzymes may run a prolonged course but are usually self-limiting. Many (but not all) cases have had allergic manifestations with fever, rash and eosinophilia. Autoantibodies are usually not present. The hepatotoxicity of ofloxacin is similar to that of other fluoroquinolones and appears to represent a class effect.
Likelihood score: A (well established but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of hepatic injury is unknown, but appears to be hypersensitivity.
Outcome and Management
The severity of ofloxacin induced liver injury ranges from mild and transient serum enzyme elevations to self-limited jaundice to acute liver failure. Recovery is usually rapid (2 to 8 weeks) after stopping the medication. Cross reactivity of the hepatic injury between different fluoroquinolones has not been well defined, but is suspected based upon the similarity of clinical patterns of injury and latency. Thus, patients should be advised to avoid further exposure to the fluoroquinolones, particularly levofloxacin which is the levorotatory isomer of ofloxacin.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Fluoroquinolones: Ciprofloxacin, Delafloxacin, Gemifloxacin, Levofloxacin, Moxifloxacin, Norfloxacin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ofloxacin – Generic, Floxin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
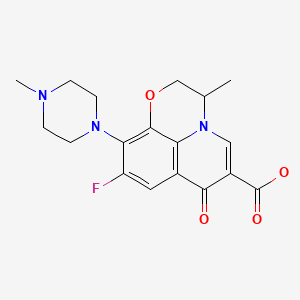
| Ofloxacin | 82419-36-1 | C18-H20-F-N3-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 March 2020
- Zimmerman HJ. Quinolones. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p 603.(Expert review of hepatotoxicity published in 1999; mentions that cinoxacin, nalidixic acid, ciprofloxacin, norfloxacin, enoxacin, and ofloxacin are associated with minor serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury).
- Moseley RH. Fluoroquinolones. Hepatotoxicity of antimicrobial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. p. 468-9.(Review of hepatotoxicity of antibiotics mentions that hepatocellular and cholestatic forms of injury have been reported due to the quinolones including cases of ductopenia, acute liver failure and death).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1015-8.(Textbook of pharmacology and therapeutics).
- Jüngst G, Mohr R. Side effects of ofloxacin in clinical trials and in postmarketing surveillance. Drugs. 1987;34 Suppl 1:144–9. [PubMed: 3325257](In postmarketing surveillance of 1.5 million patients treated with ofloxacin in Germany, 18 liver related adverse events were reported, 15 serum enzyme elevations and 3 cases of "cholestasis").
- Halkin H. Adverse effects of the fluoroquinolones. Rev Infect Dis. 1988;10 Suppl 1:S258–61. [PubMed: 3279499](Combined analysis of databases provided by manufacturers on adverse events of fluoroquinolones in approximately 30,000 persons receiving ciprofloxacin, ofloxacin, pefloxacin, norfloxacin and enoxacin found similar types and rates of adverse events among the agents, overall in 4-8%, elevated liver enzymes in 1.8-2.5%, but eosinophilia in 2.4% with ciprofloxacin and 5-19% with ofloxacin).
- Blum A. Ofloxacin-induced acute severe hepatitis. South Med J. 1991;84:1158. [PubMed: 1891746](86 year old woman with heart disease developed nausea and vomiting 10 days after completing a 14 day course of ofloxacin [bilirubin not mentioned, ALT 4160 U/L, LDH 5510 U/L, Alk P 403 U/L], with accompanying renal failure and rapid resolution; suggestive of ischemic liver injury).
- Hautekeete ML, Kockx MM, Naegels S, Holvoet JK, Hubens H, Kloppel G. Cholestatic hepatitis related to quinolones: a report of two cases. J Hepatol. 1995;23:759–60. [PubMed: 8750178](82 year old woman developed jaundice after 12 days of oral ofloxacin [bilirubin 4.5 rising to 6.5 mg/dL, ALT 65 U/L, Alk P 352 U/L, eosinophils 11%], jaundice lasting 4 weeks and then resolving; a second case was due to ciprofloxacin).
- Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf. 1995;13:343–58. [PubMed: 8652079](Review of the nature and frequency of adverse reactions to the fluoroquinolones mentions that clinically apparent liver injury is rare, but that severe and occasional fatal cases have been reported with ciprofloxacin, levofloxacin, norfloxacin and ofloxacin, and from postmarketing surveillance has estimated the frequency of clinically apparent liver injury to be 0.8 per 100,000 recipients).
- Jones SE, Smith RH. Quinolones may induce hepatitis. BMJ. 1997;314:869. [PMC free article: PMC2126221] [PubMed: 9093098](21 year old man developed jaundice 5 days after starting ofloxacin and 1 day after starting ciprofloxacin [peak bilirubin 8.2 mg/dL, AST 557 U/L, Alk P 530 U/L], resolving within 4 weeks of stopping).
- González Carro P, Huidobro ML, Zabala AP, Vicente EM. Fatal subfulminant hepatic failure with ofloxacin. Am J Gastroenterol. 2000;95:1606. [PubMed: 10894622](70 year old man developed jaundice 5 weeks after a 5 day course of ofloxacin [bilirubin 13.5 mg/dL, ALT 1382 U/L, Alk P 392 U/L], with gradual worsening and hepatic failure resulting in death 16 weeks after onset).
- Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett. 2002;127:269–77. [PubMed: 12052667](Review of the serious toxicities of the fluoroquinolones, their frequency and possible mechanisms; the hepatotoxicity of trovafloxacin became evident only after approval and over 2.5 million prescriptions worldwide with 140 reported cases of "severe hepatic reactions" despite no serious hepatotoxicity in clinical trials in several thousand patients).
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH., DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9:517–23.e3. [PMC free article: PMC3718017] [PubMed: 21356330](Among 679 cases of drug induced liver injury presenting between 2004 and 2010 at 8 US medical centers, 12 [1.8%] were attributed to fluoroquinolones including 6 cipro-, 4 moxi-, 1 levo-, and 1 gatifloxacin, but none to ofloxacin; average time to onset 4 days [range 1-39], with both hepatocellular and cholestatic enzyme patterns, seven with rash or fever, mortality limited to those with hepatocellular injury and jaundice; hepatic injury appeared to be class specific).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 29 [5.1%] attributed to quinolones).
- Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy. 2012;97:149–66. [PubMed: 22613860](Review of the clinical features, epidemiology, genetics and pathogenesis of SJS and TEN).
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389–98. [PubMed: 23619444](Systematic review of 10 case series of SJS/TEN from India identified 352 cases, among which 342 implicated a medication with most common being antimicrobials [37%], anticonvulsants [16%] and NSAIDs [16%]; fluoroquinolones accounted for 33 cases [10%], 4 of which were due to ciprofloxacin, 1 to ofloxacin and 1 to ciprofloxacin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, one due to trovafloxacin [acute liver failure], but none attributed to ciprofloxacin or other fluoroquinolones).
- Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm. 2014;71:37–43. [PubMed: 24352180](Retrospective analysis of Veterans Affairs patients receiving a fluoroquinolone [n=7862] found a higher relative risk of developing acute liver injury after receipt of ciprofloxacin compared to matched controls [adjusted odds ratio: OR=1.29], but not after receipt of levofloxacin [OR=1.16) or moxifloxacin [OR=0.98]).
- Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015; 148: 1353-61. e3. [PMC free article: PMC4446162] [PubMed: 25733099](Analysis of Kaiser Permanente health care database from 2004 to 2011 identified 62 patients with suspected acute liver failure, 32 [52%] of whom had a presumed drug etiology, the most common being acetaminophen [18: 56%] and various herbal products [5: 16%], with single instances attributed to imatinib, simvastatin, leflunomide, isoniazid and valproate, but none to fluoroquinolones).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 38 cases [4%] were attributed to fluoroquinolones, including 16 due to ciprofloxacin [the 8th most common prescription drug cause], 13 due to levofloxacin and 8 to moxifloxacin).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss of whom 94% developed evidence of chronic liver injury suggestive of vanishing bile duct syndrome, 2 of which were due to fluoroquinolones, 1 to moxifloxacin and 1 levofloxacin).
- Comparison table: some systemic fluoroquinolones. Med Lett Drugs Ther. 2018;60:e57–e58. [PubMed: 29635268](Table comparing 4 fluoroquinolones [cipro-, levo-, dela- and moxifloxacin] mentions that ALT and AST elevations are a class adverse event).
- Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One. 2019;14:e0216029. [PMC free article: PMC6485715] [PubMed: 31026286](Systematic review of observational studies on safety of fluoroquinolones concluded that due to lack of published literature, health service and costs could not be evaluated).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review The clinical treatment of bacterial keratitis: A review of drop instillation regimes.[Cont Lens Anterior Eye. 2022]Review The clinical treatment of bacterial keratitis: A review of drop instillation regimes.Pearce JG, Essex RW, Maddess T. Cont Lens Anterior Eye. 2022 Dec; 45(6):101725. Epub 2022 Jun 17.
- In vitro activity of fluoroquinolones against ocular bacterial isolates in São Paulo, Brazil.[Cornea. 2007]In vitro activity of fluoroquinolones against ocular bacterial isolates in São Paulo, Brazil.Oliveira AD, D'Azevedo PA, Francisco W. Cornea. 2007 Feb; 26(2):194-8.
- Fluoroquinolone resistance in bacterial isolates from ocular infections: Trend in antibiotic susceptibility patterns between 2005-2020.[Indian J Ophthalmol. 2022]Fluoroquinolone resistance in bacterial isolates from ocular infections: Trend in antibiotic susceptibility patterns between 2005-2020.Chatterjee S, Agrawal D, Gomase SN, Parchand SM, Gangwe AB, Mishra M. Indian J Ophthalmol. 2022 Dec; 70(12):4391-4398.
- Review A Literature-Based Review and Analysis of the Pharmacodynamics of the Dose Frequency of Topical 0.3% Ciprofloxacin and 0.3% Ofloxacin in the Day-1 Treatment of Bacterial Keratitis.[J Ocul Pharmacol Ther. 2023]Review A Literature-Based Review and Analysis of the Pharmacodynamics of the Dose Frequency of Topical 0.3% Ciprofloxacin and 0.3% Ofloxacin in the Day-1 Treatment of Bacterial Keratitis.Pearce JG, Naunton M, Maddess T. J Ocul Pharmacol Ther. 2023 Jan-Feb; 39(1):17-26. Epub 2022 Nov 29.
- Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses.[Eur J Clin Pharmacol. 1999]Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses.Hehl EM, Beck R, Luthard K, Guthoff R, Drewelow B. Eur J Clin Pharmacol. 1999 Jun; 55(4):317-23.
- Ofloxacin - LiverToxOfloxacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...