NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Delafloxacin is a fourth generation fluoroquinolone with expanded activity against gram-positive bacteria as well as atypical pathogens. Delafloxacin has been linked to mild ALT elevations during therapy, but has yet to be linked to instances of idiosyncratic acute liver injury with symptoms and jaundice as have been described with other fluoroquinolones.
Background
Delafloxacin (del" a flox' a sin) is a fourth generation fluoroquinolone with expanded activity against gram-positive bacteria including multidrug resistant strains of Streptococcus pneumoniae. Like other fluoroquinolones, delafloxacin is active against a wide range of aerobic gram-positive and gram-negative organisms. The quinolones are believed to act by inhibition of bacterial DNA gyrase and topoisomerase IV that are required for synthesis of bacterial mRNAs (transcription) and DNA replication. In contrast, DNA gyrases are not present in human [and other eukarotic] cells and the equivalent topoisomerases are not sensitive to fluoroquinolone inhibition. Delafloxacin was approved for use in the United States in 2018 and is available under the commercial name Baxdela. Current indications are limited to skin and skin structure infections due to sensitive organisms. Delafloxacin is available in both oral and parenteral formulations as 450 mg tablets and as 300 mg of a lyophilized powder in a single dose vial for reconstitution. The recommended regimen is an oral dose of 450 mg every 12 hours or by intravenous infusion every 12 hours for 5 to 14 days. Common side effects include gastrointestinal upset, nausea, diarrhea, headache, skin rash and allergic reactions. Less common, but more severe side effects of delafloxacin that are shared by other fluoroquinolones include seizures, hallucinations, peripheral neuropathy, tendon rupture, severe hypersensitivity reactions, Stevens Johnson syndrome, angioedema, photosensitivity and Clostridium difficile-associated diarrhea.
Hepatotoxicity
Delafloxacin, like other fluoroquinolones, is associated with a low rate (3% to 4%) of serum enzyme elevations during therapy. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. ALT elevations above 5 times the upper limit of normal occur in 1% or less of subjects. Although delafloxacin may not have been clearly linked to cases of clinically apparent liver injury, the other fluoroquinolones, such as ciprofloxacin, levofloxacin and moxifloxacin, rank among the 25 most common causes of drug induced liver injury in many case series. Estimates of the frequency of liver injury from fluoroquinolones have been 1:15,000 to 1:25,000 exposed persons. Delafloxacin has been in clinical use for a short time only, but is likely to have a similar frequency and pattern of liver injury as the other fluoroquinolones.
The typical presentation of fluoroquinolone associated liver injury is with a short latency (1 day to 3 weeks) and abrupt onset with nausea, fatigue, abdominal pain and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic, cases with the shorter times to onset usually being more hepatocellular. In addition, the onset of illness may occur a few days after the medication is stopped. Many (but not all) cases have prominent allergic manifestations with fever and rash, and the liver injury may occur in the context of a generalized hypersensitivity reaction. Autoantibodies are usually not present. Most reported cases of liver injury from fluoroquinolones have been mild and self-limited, with recovery in 4 to 8 weeks from onset. However, the fatality rate of cases with jaundice has been greater than 10%. In addition, cases with a cholestatic pattern of serum enzymes may run a prolonged course and, in rare instances, have progressed to chronic vanishing bile duct syndrome leading to liver failure. Nevertheless, delafloxacin is a relatively recently introduced antibiotic and has yet to be convincingly linked to instances of acute hepatitis or jaundice.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The cause of hepatic injury from the fluoroquinolones is unknown but appears to be hypersensitivity.
Outcome and Management
Mild-to-moderate injury from fluoroquinolone induced hepatitis should be followed by full recovery within 4 to 8 weeks. Cases of acute liver failure with death or need for liver transplantation have been reported, nor have chronic cases with bile duct loss. Cross reactivity of the hepatic injury between different fluoroquinolones has not been demonstrated but is suspected based upon the similarity of clinical patterns of injury and latency. Thus, patients who developed clinically apparent liver injury from a fluoroquinolone should be advised to avoid delafloxacin. Monitoring of liver tests during therapy is likely to be ineffective as the typical course of treatment is only 5 to 10 days.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Fluoroquinolones: Ciprofloxacin, Gemifloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Delafloxacin – Baxdela®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
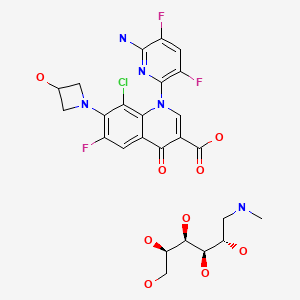
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Delafloxacin Meglumine | 352458-37-8 | C18-H12-Cl-F3-N4-O4.C7-H17-N-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 March 2020
- Zimmerman HJ. Quinolones. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p 603.(Expert review of hepatotoxicity published in 1999; mentions that cinoxacin, nalidixic acid, ciprofloxacin, norfloxacin, enoxacin, and ofloxacin are associated with minor serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury; delafloxacin is not discussed).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics; mentions that hepatocellular and cholestatic forms of injury have been reported due to the fluoroquinolones including cases of ductopenia, acute liver failure and death).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1015-8.(Textbook of pharmacology and therapeutics).
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH., DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9:517–523.e3. [PMC free article: PMC3718017] [PubMed: 21356330](Among 679 cases of drug induced liver injury presenting between 2004 and 2010 at 8 US medical centers, 12 [1.8%] were attributed to fluoroquinolones including 6 cipro-, 4 moxi-, 1 levo-, and 1 gatifloxacin; the average time to onset was 4 days [range 1-39], with both hepatocellular and cholestatic enzyme patterns, 7 with rash or fever, mortality was limited to those with hepatocellular injury and jaundice; hepatic injury was class specific; moxifloxacin cases included 3 men, 1 woman, ages 45-71 years, onset after 1-7 days, 1 with fever and 2 with rash [bilirubin 0.9-5.3 mg/dL, ALT 220-1311 U/L, Alk P 253-837 U/L], 1 patient developed vanishing bile duct syndrome and underwent liver transplantation: Case 1).
- Paterson JM, Mamdani MM, Manno M, Juurlink DN., Canadian Drug Safety and Effectiveness Research Network. Fluoroquinolone therapy and idiosyncratic acute liver injury: a population-based study. CMAJ. 2012;184:1565–70. [PMC free article: PMC3470619] [PubMed: 22891208](Population based, case control study of antibiotic exposure and subsequent hospitalization for liver injury within 30 days in elderly Canadian outpatients found weak associations with ciprofloxacin [adjusted odds ratio 1.56]), levofloxacin [2.06] and moxifloxacin [2.44], but not clarithromycin or cefuroxime).
- Hayashi PH, Chalasani NP. Liver injury in the elderly due to fluoroquinolones: should these drugs be avoided? CMAJ. 2012;184:1555–6. [PMC free article: PMC3470615] [PubMed: 22891207](Editorial in response to Paterson [2012] stressing the low absolute risk of liver injury from the fluoroquinolones [4-9 per 100,000 exposures]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 26 due to antibiotics, but none were attributed to fluoroquinolones).
- Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm. 2014;71:37–43. [PubMed: 24352180](Retrospective analysis of Veterans Affairs patients receiving a fluoroquinolone [n=7862] found a higher relative risk of developing acute liver injury after receipt of ciprofloxacin compared to matched controls [adjusted odds ratio: OR=1.29], but not after receipt of levofloxacin [OR=1.16) or moxifloxacin [OR=0.98]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 38 cases [4%] were attributed to fluoroquinolones, including 16 due to ciprofloxacin [the 8th most common prescription drug cause], 13 due to levofloxacin and 8 to moxifloxacin).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss, of whom 94% developed evidence of chronic liver injury suggestive of vanishing bile duct syndrome, 2 of which were due to fluoroquinolones, 1 moxifloxacin and 1 levofloxacin).
- O'Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis. 2015;30:67–73. [PubMed: 25448332](Among 150 patients with complicated skin or skin structure infections treated with 1 of 2 doses of delafloxacin or tigercycline for 5-14 days, clinical efficacy and adverse event rates were similar; ALT elevations occurring in 3% of delafloxacin, but in none of tigecycline treated subjects).
- Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, Ling R, et al. PROCEED Study Group. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: A Phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72:3471–80. [PMC free article: PMC5890686] [PubMed: 29029278](Among 660 patients with acute bacterial skin or skin structure infections treated with delafloxacin or the combination of vancomycin and aztreonam, clinical response and adverse event rates were similar, ALT elevations above 5 times ULN occurred in 0.9% vs 1.5% of subjects, and no patient developed clinically apparent liver injury with jaundice).
- Markham A. Delafloxacin: first global approval. Drugs. 2017;77:1481–6. [PMC free article: PMC6208769] [PubMed: 28748399](Review of the mechanism of action, pharmacology, clinical efficacy and safety of delafloxacin; mentions that aminotransferase elevations occur in 3-4% of treated patients, but cases of clinically apparent liver injury with jaundice were not observed in the prelicensure studies).
- Delafloxacin (Baxdela)--a new fluoroquinolone antibiotic. Med Lett Drugs Ther. 2018;60(1543):49–51. [PubMed: 29635263](Concise review of the mechanism of action, clinical efficacy, safety and costs of delafloxacin shortly after its approval for use in the US; mentions adverse side effects of aminotransferase elevations [3%] and that it, like other fluoroquinolones, has a boxed warning about tendinitis, tendon rupture, peripheral neuropathy and CNS effects, and that it can also lead to C. difficile diarrhea).
- O'Riordan W, McManus A, Teras J, Poromanski I, Cruz-Saldariagga M, Quintas M, Lawrence L, et al. PROCEED Study Group. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. Clin Infect Dis. 2018;67:657–66. [PMC free article: PMC6093995] [PubMed: 29518178](Among 850 patients with acute bacterial skin or skin structure infections treated with delafloxacin or the combination of vancomycin and aztreonam for 5-14 days, clinical efficacy and overall adverse event rates were similar in the two groups, no patient developed clinically apparent liver injury with jaundice and ALT elevations above 5 times ULN occurred in 1.2% vs 1.9% of subjects).
- Comparison table: some systemic fluoroquinolones. Med Lett Drugs Ther. 2018;60:e57–e58. [PubMed: 29635268](Table comparing 4 fluoroquinolones [cipro-, levo-, dela- and moxifloxacin] mentions that ALT and AST elevations are a class adverse event).
- Mavros MN, Theochari NA, Kyriakidou M, Economopoulos KP, Sava JA, Falagas ME. Fluoroquinolone-based versus β-lactam-based regimens for complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2019;53:746–54. [PubMed: 30639629](Systematic review of controlled trials of fluoroquinolones versus β-lactam-based antibiotic regimens found similar rates of efficacy and adverse events; no discussion of ALT elevations or liver related toxicities).
- Kuula LSM, Viljemaa KM, Backman JT, Blom M. Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One. 2019;14:e0216029. [PMC free article: PMC6485715] [PubMed: 31026286](Systematic review of observational studies on safety of fluoroquinolones concluded that due to lack of published literature, health service and costs could not be evaluated).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Moxifloxacin.[LiverTox: Clinical and Researc...]Review Moxifloxacin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone.[Pharmacotherapy. 2018]Review What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone.Cho JC, Crotty MP, White BP, Worley MV. Pharmacotherapy. 2018 Jan; 38(1):108-121. Epub 2017 Nov 23.
- In Vitro Activity of Delafloxacin and Microbiological Response against Fluoroquinolone-Susceptible and Nonsusceptible Staphylococcus aureus Isolates from Two Phase 3 Studies of Acute Bacterial Skin and Skin Structure Infections.[Antimicrob Agents Chemother. 2...]In Vitro Activity of Delafloxacin and Microbiological Response against Fluoroquinolone-Susceptible and Nonsusceptible Staphylococcus aureus Isolates from Two Phase 3 Studies of Acute Bacterial Skin and Skin Structure Infections.McCurdy S, Lawrence L, Quintas M, Woosley L, Flamm R, Tseng C, Cammarata S. Antimicrob Agents Chemother. 2017 Sep; 61(9). Epub 2017 Aug 24.
- Efficacy of Delafloxacin versus Moxifloxacin against Bacterial Respiratory Pathogens in Adults with Community-Acquired Bacterial Pneumonia (CABP): Microbiology Results from the Delafloxacin Phase 3 CABP Trial.[Antimicrob Agents Chemother. 2...]Efficacy of Delafloxacin versus Moxifloxacin against Bacterial Respiratory Pathogens in Adults with Community-Acquired Bacterial Pneumonia (CABP): Microbiology Results from the Delafloxacin Phase 3 CABP Trial.McCurdy S, Keedy K, Lawrence L, Nenninger A, Sheets A, Quintas M, Cammarata S. Antimicrob Agents Chemother. 2020 Feb 21; 64(3). Epub 2020 Feb 21.
- Review Delafloxacin: A Review in Community-Acquired Pneumonia.[Drugs. 2022]Review Delafloxacin: A Review in Community-Acquired Pneumonia.Lee A, Lamb YN, Shirley M. Drugs. 2022 Jun; 82(8):913-923. Epub 2022 Jun 16.
- Delafloxacin - LiverToxDelafloxacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...