NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nilutamide is a first generation, oral nonsteroidal antiandrogen similar in structure to flutamide that is used in the therapy of prostate cancer. Nilutamide is associated with a low rate of serum aminotransferase elevations during therapy and with rare instances of clinically apparent, acute liver injury.
Background
Nilutamide (nye loo' ta mide) is an anilide nonsteroidal antiandrogen that blocks the binding of endogenous androgens to intracellular androgen receptors blocking their effects. Nilutamide has been shown to be effective in reducing pain and disease progression in metastatic prostate cancer when administered in conjunction with orchiectomy or other antiandrogen agents such as luteinizing hormone releasing hormone (LHRH) agonists. Nilutamide was approved for use in the United States in 1996. Current indications are limited to the therapy of metastatic prostate cancer in combination with orchiectomy. Nilutamide is available generically and under the trade name Nilandron in 150 mg tablets, and the typically recommended dose is 300 mg daily starting at the time of orchiectomy with reduction of the dose to 150 mg daily one month later. Nilutamide is not approved for nor recommended for use in hyperandrogenic states such as hirsutism or acne. Common side effects include dizziness, impaired vision, fatigue, nausea, anorexia and weight loss. An uncommon but potentially serious adverse event is interstitial pneumonitis that occurs in approximately 2% of treated patients and that can progress to respiratory failure and death.
Hepatotoxicity
In large registration clinical trials, ALT elevations occurred in 8% (range 2% to 33%) of patients during nilutamide therapy. The elevations were usually mild, asymptomatic and transient, requiring drug discontinuation in only 1% of treated patients. In rare instances, clinically apparent acute liver injury has occurred during nilutamide therapy, but the number of published cases are few, and the agent appears to be less hepatotoxic than flutamide. Nevertheless, fatal cases have been reported (Case 1). In reported cases, the latency to onset averaged 2 to 4 months and the clinical pattern of enzyme elevations was typically hepatocellular, thus largely resembling flutamide induced liver injury. Signs of hypersensitivity and autoimmunity were not common.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of nilutamide hepatotoxicity is unknown, but toxic metabolites of the agent may induce oxidative stress or interfere with mitochondrial function.
Outcome and Management
The mild ALT elevations during nilutamide therapy are usually self-limiting even with continuation of the medication. The rare instances of clinically apparent liver injury are usually self-limiting, but several fatal instances have been reported. Monitoring of liver tests is recommended before starting treatment and at regular intervals thereafter, particularly during the first 4 months of treatment and therapy stopped if symptoms, jaundice or marked serum aminotransferase elevations arise. Rechallenge should be avoided. Patients with nilutamide hepatotoxicity should probably not receive flutamide and be given bicalutamide with caution.
Drug Class: Antineoplastic Agents, Antiandrogens
CASE REPORTS
Case 1. Acute liver failure from nilutamide therapy.(1)
A 65 year old man with prostate cancer developed jaundice 8 weeks after starting nilutamide for prostate cancer. Nilutamide was stopped promptly, but he continued to worsen and developed mental confusion and was admitted to a hospital. On examination, he was markedly jaundiced and had stage 2 hepatic encephalopathy (asterixis and confusion). His past medical history was negative for liver disease and he had no risk factors for viral hepatitis and rarely drank alcohol. He had been diagnosed with prostate cancer and underwent orchiectomy 3 months previously and was treated postoperatively for 3 weeks with norfloxacin and prednisolone. Liver tests were reported to be normal before he was started on nilutamide (150 mg/day) and leuprolide (an LHRH analog: 3.75 mg intramuscularly each month). At the time that nilutamide was stopped 8 weeks later, serum bilirubin was 7.5 mg/dL, and it rose rapidly thereafter (Table). Tests for hepatitis A, B and C, EBV and CMV as well as for autoantibodies were negative. Abdominal ultrasound showed no evidence of biliary disease. He developed progressive mental obtundation, coagulopathy and, not being a candidate for emergency liver transplantation, died 16 days after onset of jaundice. Post mortem liver biopsy showed massive necrosis with minimal fibrosis.
Key Points
| Medication: | Nilutamide, 150 mg daily for 8 weeks |
|---|---|
| Pattern: | Hepatocellular |
| Severity: | 5+ (acute liver failure and death) |
| Latency: | 8 weeks |
| Recovery: | None |
| Other medications: | Leuprolide. Prednisolone and norfloxacin 2 months previously |
Laboratory Values
Comment
Despite stopping nilutamide when jaundice was first identified, this patient developed progressive hepatic failure and died within weeks of onset of liver injury. The latency (2 months), pattern of serum enzyme elevations (markedly hepatocellular), and lack of other obvious causes for liver injury (viral hepatitis, alcoholism, biliary disease, ischemia) points strongly to the role of nilutamide. The overall clinical characteristics of nilutamide hepatotoxicity resemble those of flutamide, but clinically apparent liver disease appears to be less common.
Case 2. Interstitial pneumonitis and mild elevations in serum enzymes during nilutamide therapy.(2)
A 69 year old man with prostate cancer developed shortness of breath and liver enzyme abnormalities 2 months after starting the combination of nilutamide and an luteinizing hormone releasing hormone (LHRH) analog. He had noticed progressive shortness of breath over the previous 4 weeks and was found to have tachypnea and abnormal chest x-ray findings suggestive of interstitial pneumonitis. Nilutamide was stopped. At the same time, blood test results showed mild elevations in ALT and alkaline phosphatase in comparison to pretreatment values. However, serum bilirubin levels were normal (Table). There was no eosinophilia and no signs of chronic liver disease or hepatic decompensation. Tests for viral hepatitis and autoantibodies were negative. A lung biopsy showed interstitial pneumonitis. In follow up, serum enzyme levels fell to pretreatment levels. He recovered slowly from the pulmonary complication. Flutamide was started after all test results returned to normal and was continued with no apparent toxic effects.
Key Points
| Medication: | Nilutamide, 100 mg twice daily |
|---|---|
| Pattern: | Hepatocellular |
| Severity: | 1+ (enzyme elevations without jaundice) |
| Latency: | 2 months |
| Recovery: | 75 days |
| Other medications: | LHRH agonist |
Laboratory Values
Comment
Interstitial pneumonitis is an uncommon but well established complication of nilutamide therapy. Symptoms and signs reverse slowly and only partially after therapy is stopped. This patient was also found to have minor ALT elevations but not clinically apparent liver disease during therapy. In most situations, such minor ALT elevations can be monitored without dose modification. The fact that this patient tolerated flutamide therapy without subsequent evidence of liver injury does not indicate that there is no cross reactivity in instances of clinically apparent hepatotoxicity between these two agents. Switching therapy to flutamide is not recommended for patients with clinically apparent liver injury from nilutamide.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nilutamide – Generic, Nilandron®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nilutamide | 63612-50-0 | C12-H10-F3-N3-O4 |
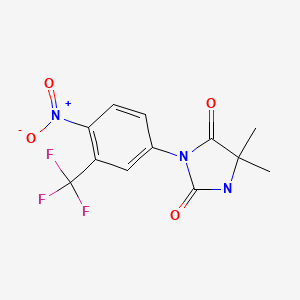
|
CITED REFERENCES
- 1.
- Marty F, Godart D, Doermann F, Mérillon H. Gastroenterol Clin Biol. 1996;20:710–1. [Fatal fulminating hepatitis caused by nilutamide. A new case] French. [PubMed: 8977826]
- 2.
- Gomez JL, Dupont A, Cusan L, Tremblay M, Tremblay M, Labrie F. Simultaneous liver and lung toxicity related to the nonsteroidal antiandrogen nilutamide (Anandron): a case report. Am J Med. 1992;92:563–6. [PubMed: 1580304]
ANNOTATED BIBLIOGRAPHY
References updated: 15 March 2023
Abbreviations: LHRH, luteinizing hormone releasing hormone; PSA, prostate-specific antigen.
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, p. 699.(Expert review of hepatotoxicity published in 1999, mentions that flutamide has led to instances of severe hepatic necrosis and fulminant hepatic failure).
- DeLeve LD. Antiandrogens. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 612-3.(Textbook on drug induced liver injury mentions that nilutamide has been implicated in causing at least four cases of acute hepatitis, two of which were fatal).
- Moy B, Lee RJ, Smith M. Hormone therapy in prostate cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1763-9.(Textbook of pharmacology and therapeutics; discusses the role of androgen receptor blockers in prostate cancer including bicalutamide, flutamide and nilutamide).
- Hammel P, Ducreux M, Bismuth E, et al. Gastroenterol Clin Biol. 1991;15:557. [Nilutamide-induced acute hepatitis] French. [PubMed: 1916139](68 year old man with prostate cancer developed itching and then jaundice 4 months after starting nilutamide [bilirubin 8.2 mg/dL, ALT 22 times and Alk P 2 times ULN, eosinophils 800/μL], biopsy showed submassive necrosis; rapid improvement upon stopping with ultimate full recovery).
- Gomez JL, Dupont A, Cusan L, Tremblay M, Tremblay M, Labrie F. Simultaneous liver and lung toxicity related to the nonsteroidal antiandrogen nilutamide (Anandron): a case report. Am J Med. 1992;92:563–6. [PubMed: 1580304](69 year old man with prostate cancer developed pulmonary toxicity [interstitial pneumonitis] 2 months after starting nilutamide with concurrent minor enzyme abnormalities [bilirubin 0.50 mg/dL, ALT 96 U/L, Alk P 150 U/L], which resolved rapidly with stopping: Case 2).
- Pescatore P, Hammel P, Durand F, et al. Gastroenterol Clin Biol. 1993;17:499–501. [Fatal fulminant hepatitis induced by nilutamide (Anandron)] French. [PubMed: 8243938](69 year old man with prostate cancer underwent orchiectomy and developed jaundice 8 weeks after starting nilutamide [bilirubin 13.9 mg/dL, ALT 45 times ULN], with progressive liver failure and death within 2 weeks; autopsy showed massive necrosis).
- Bertagna C, De Géry A, Hucher M, François JP, Zanirato J. Efficacy of the combination of nilutamide plus orchidectomy in patients with metastatic prostatic cancer. A meta-analysis of seven randomized double-blind trials (1056 patients). Br J Urol. 1994;73:396–402. [PubMed: 8199827](Review of 7 randomized clinical trials of nilutamide in 1191 patients with prostate cancer showed improved pain and objective evidence of disease but not survival compared to placebo; no mention of hepatotoxicity).
- Marty F, Godart D, Doermann F, Mérillon H. Gastroenterol Clin Biol. 1996;20:710–1. [Fatal fulminating hepatitis caused by nilutamide. A new case] French. [PubMed: 8977826](65 year old man with prostate cancer developed jaundice 8 weeks after starting nilutamide [bilirubin 9.3 mg/dL, ALT 2360 U/L, Alk P 231 U/L], with rapid progression to liver failure and death 3 weeks later: Case 1).
- Dijkman GA, Janknegt RA, De Reijke TM, Debruyne FM. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J Urol. 1997;158:160–3. [PubMed: 9186345](Long term follow up of randomized clinical trials of nilutamide vs placebo after orchiectomy in 457 patients with prostate cancer; overall survival improved by 7 months, no new long term drug specific serious adverse event, no mention of hepatotoxicity).
- McLeod DG. Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist. 1997;2:18–27. [PubMed: 10388026](Overview of the safety and side effects of the nonsteroidal antiandrogens; ALT elevations occur in 2-33% receiving nilutamide compared to 4-62% with flutamide; 5 cases of clinically apparent hepatitis have been reported due to nilutamide, compared to more than 50 with flutamide).
- Edouard A, Robinel R, Rat C, Lombard F, Lorinet C, Escarmant P. Gastroenterol Clin Biol. 2003;27:1170–1. [Fatal fulminating hepatitis induced by nilutamide] French. [PubMed: 14770126](67 year old man with prostate cancer developed jaundice 3 months after starting nilutamide [bilirubin 14.2 mg/dL, ALT 35 times and Alk P 1.5 times ULN, protime 42%], with progressive hepatic failure and death 4 weeks later).
- Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A. Hepatotoxicity induced by antiandrogens: a review of the literature. Urol Int. 2004;73:289–95. [PubMed: 15604569](Systematic review of the literature from the Spanish pharmacovigilance group; 21 reports on hepatotoxicity of cyproterone, 46 flutamide, 4 nilutamide and only 1 bicalutamide; 6 cases of hepatocellular carcinoma linked to cyproterone therapy).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but no case was attributed to an antiandrogen, perhaps because patients with prostate cancer would not be eligible for liver transplantation).
- Manso G, Thole Z, Salgueiro E, Revuelta P, Hidalgo A. Spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmacovigilance system. Pharmacoepidemiol Drug Saf. 2006;15:253–9. [PubMed: 16294367](Analysis of spontaneous reporting to Spanish pharmacovigilance system found 88 cases of flutamide, 11 bicalutamide and 15 cyproterone hepatotoxicity, latency 3-6 months; 2 fatalities, both from flutamide; nilutamide not used in Spain).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports; flutamide ranked 11th with a total of 59 cases).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to an antiandrogen).
- Merwat SN, Kabbani W, Adler DG. Fulminant hepatic failure due to nilutamide hepatotoxicity. Dig Dis Sci. 2009;54:910–3. [PubMed: 18688719](76 year old man with prostate cancer developed abdominal pain followed by jaundice 6 weeks after starting nilutamide [bilirubin 25.8 mg/dL, ALT 1793 U/L, Alk P 231 U/L, INR 1.7], followed by progressive hepatic and multiorgan failure and death).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to antiandrogen therapies).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to bicalutamide, but none to nilutamide or other antiandrogens).
- Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, et al. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J Urol. 2018;200:956–966. [PubMed: 29730201](Review of the development of antiandrogen therapies of prostate cancer starting with discovery of the androgen sensitive nature of prostate cancer, the effects of orchiectomy, followed by the development of androgen receptor antagonists, first generation agents flutamide and nilutamide, second generation agent bicalutamide and third generation agents enzalutamide, apalutamide and darolutamide that have more potent androgen receptor inhibition).
- Rashid M, Shamshavali K, Chhabra M. Efficacy and safety of nilutamide in patients with metastatic prostate cancer who underwent orchiectomy: a systematic review and metaanalysis. Curr Clin Pharmacol. 2019;14:108–115. [PMC free article: PMC7040499] [PubMed: 30648519](Systematic review identified 5 controlled trials of nilutamide in 1637 patients with prostate cancer, that showed improvements in both overall and progression free survival with nilutamide compared to placebo and adverse events included delayed adaption to darkness, blurred vision, alcohol intolerance, hot flashes, nausea, respiratory disorders and serum enzyme elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Antiandrogens.[LiverTox: Clinical and Researc...]Review Antiandrogens.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nilutamide: an antiandrogen for the treatment of prostate cancer.[Ann Pharmacother. 1997]Review Nilutamide: an antiandrogen for the treatment of prostate cancer.Dole EJ, Holdsworth MT. Ann Pharmacother. 1997 Jan; 31(1):65-75.
- Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer.[Oncologist. 1997]Tolerability of Nonsteroidal Antiandrogens in the Treatment of Advanced Prostate Cancer.McLeod DG. Oncologist. 1997; 2(1):18-27.
- Review Bicalutamide.[LiverTox: Clinical and Researc...]Review Bicalutamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Flutamide.[LiverTox: Clinical and Researc...]Review Flutamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Nilutamide - LiverToxNilutamide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...